Identification of the Anterior Nucleus of the Ansa Lenticularis in Birds As
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Imaging of Ventral Thalamic Nuclei
ORIGINAL RESEARCH MR Imaging of Ventral Thalamic Nuclei K. Yamada BACKGROUND AND PURPOSE: The Vim and VPL are important target regions of the thalamus for DBS. K. Akazawa Our aim was to clarify the anatomic locations of the ventral thalamic nuclei, including the Vim and VPL, on MR imaging. S. Yuen M. Goto MATERIALS AND METHODS: Ten healthy adult volunteers underwent MR imaging by using a 1.5T S. Matsushima whole-body scanner. The subjects included 5 men and 5 women, ranging in age from 23 to 38 years, with a mean age of 28 years. The subjects were imaged with STIR sequences (TR/TE/TI ϭ 3200 ms/15 A. Takahata ms/120 ms) and DTI with a single-shot echo-planar imaging technique (TR/TE ϭ 6000 ms/88 ms, M. Nakagawa b-value ϭ 2000 s/mm2). Tractography of the CTC and spinothalamic pathway was used to identify the K. Mineura thalamic nuclei. Tractography of the PT was used as a reference, and the results were superimposed T. Nishimura on the STIR image, FA map, and color-coded vector map. RESULTS: The Vim, VPL, and PT were all in close contact at the level through the ventral thalamus. The Vim was bounded laterally by the PT and medially by the IML. The VPL was bounded anteriorly by the Vim, laterally by the internal capsule, and medially by the IML. The posterior boundary of the VPL was defined by a band of low FA that divided the VPL from the pulvinar. CONCLUSIONS: The ventral thalamic nuclei can be identified on MR imaging by using reference structures such as the PT and the IML. -

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Magnetic Resonance Imaging Techniques for Visualization of the Subthalamic Nucleus
J Neurosurg 115:971–984, 2011 Magnetic resonance imaging techniques for visualization of the subthalamic nucleus A review ELLEN J. L. BRUNENbeRG, M.SC.,1 BRAM PLATEL, PH.D.,2 PAUL A. M. HOFMAN, PH.D., M.D.,3 BART M. TER HAAR ROmeNY, PH.D.,1,4 AND VeeRLE VIsseR-VANdeWALLE, PH.D., M.D.5,6 1Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven; Departments of 3Radiology, 4Biomedical Engineering, and 5Neurosurgery, and 6Maastricht Institute for Neuromodulative Development, Maastricht University Medical Center, Maastricht, The Netherlands; and 2Fraunhofer MEVIS, Bremen, Germany The authors reviewed 70 publications on MR imaging–based targeting techniques for identifying the subtha- lamic nucleus (STN) for deep brain stimulation in patients with Parkinson disease. Of these 70 publications, 33 presented quantitatively validated results. There is still no consensus on which targeting technique to use for surgery planning; methods vary greatly between centers. Some groups apply indirect methods involving anatomical landmarks, or atlases incorporating ana- tomical or functional data. Others perform direct visualization on MR imaging, using T2-weighted spin echo or inver- sion recovery protocols. The combined studies do not offer a straightforward conclusion on the best targeting protocol. Indirect methods are not patient specific, leading to varying results between cases. On the other hand, direct targeting on MR imaging suffers from lack of contrast within the subthalamic region, resulting in a poor delineation of the STN. These defi- ciencies result in a need for intraoperative adaptation of the original target based on test stimulation with or without microelectrode recording. It is expected that future advances in MR imaging technology will lead to improvements in direct targeting. -

Cortex and Thalamus Lecture.Pptx
Cerebral Cortex and Thalamus Hyperbrain Ch 2 Monica Vetter, PhD January 24, 2013 Learning Objectives: • Anatomy of the lobes of the cortex • Relationship of thalamus to cortex • Layers and connectivity of the cortex • Vascular supply to cortex • Understand the location and function of hypothalamus and pituitary • Anatomy of the basal ganglia • Primary functions of the different lobes/ cortical regions – neurological findings 1 Types of Cortex • Sensory (Primary) • Motor (Primary) • Unimodal association • Multimodal association - necessary for language, reason, plan, imagine, create Note: • Gyri • Sulci • Fissures • Lobes 2 The Thalamus is highly interconnected with the cerebral cortex, and handles most information traveling to or from the cortex. “Specific thalamic Ignore nuclei” – have well- names of defined sensory or thalamic nuclei for motor functions now - A few Other nuclei have will more distributed reappear later function 3 Thalamus Midbrain Pons Limbic lobe = cingulate gyrus Structure of Neocortex (6 layers) white matter gray matter Pyramidal cells 4 Connectivity of neurons in different cortical layers Afferents = inputs Efferents = outputs (reciprocal) brainstem etc Eg. Motor – Eg. Sensory – more efferent more afferent output input Cortico- cortical From Thalamus To spinal cord, brainstem etc. To Thalamus Afferent and efferent connections to different ….Depending on whether they have more layers of cortex afferent or efferent connections 5 Different areas of cortex were defined by differences in layer thickness, and size and -

MRI Atlas of the Human Deep Brain Jean-Jacques Lemaire
MRI Atlas of the Human Deep Brain Jean-Jacques Lemaire To cite this version: Jean-Jacques Lemaire. MRI Atlas of the Human Deep Brain. 2019. hal-02116633 HAL Id: hal-02116633 https://hal.uca.fr/hal-02116633 Preprint submitted on 1 May 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License MRI ATLAS of the HUMAN DEEP BRAIN Jean-Jacques Lemaire, MD, PhD, neurosurgeon, University Hospital of Clermont-Ferrand, Université Clermont Auvergne, CNRS, SIGMA, France This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA. Terminologia Foundational Model Terminologia MRI Deep Brain Atlas NeuroNames (ID) neuroanatomica usages, classical and french terminologies of Anatomy (ID) Anatomica 1998 (ID) 2017 http://fipat.library.dal.ca In -
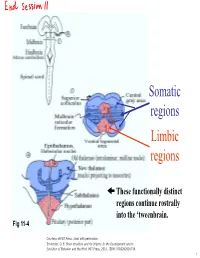
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Neuromodulation in Eating Disorders and Obesity: a Promising Way of Treatment?
Journal name: Neuropsychiatric Disease and Treatment Article Designation: Review Year: 2018 Volume: 14 Neuropsychiatric Disease and Treatment Dovepress Running head verso: Jáuregui-Lobera and Martínez-Quiñones Running head recto: Neuromodulation in eating disorders and obesity open access to scientific and medical research DOI: 180231 Open Access Full Text Article REVIEW Neuromodulation in eating disorders and obesity: a promising way of treatment? Ignacio Jáuregui-Lobera1 Abstract: Neuromodulation can affect the functioning of the central nervous system (CNS), José V Martínez-Quiñones2 and emotional/eating behavior is an exciting facet of that functioning. Therefore, it would be possible to offer an alternative (or complement) treatment to psychotropic medications and 1Department of Molecular Biology and Biochemical Engineering, different psychological and nutritional approaches to both eating disorders (EDs) and obe- University of Pablo de Olavide of sity. Although there are a number of publications in these areas, a systematic review has not Seville, Seville, Spain; 2Department of Neurosurgery, Mutua de been conducted to date. Abstracts, letters, conference reports, dissertations, and reviews were Accidentes de Zaragoza (Servicio de excluded. Clinical trials and controlled human clinical trials were filtered and included in this Neurocirugía), Zaragoza, Spain study. Articles included were based on the population suffering from anorexia nervosa, bulimia nervosa, binge ED, overweight, and obesity. No restrictions were placed on the sample size. Only trials investigating the effect of neuromodulation by means of deep brain stimulation (DBS), transcranial direct current stimulation (tDCS), and transcranial magnetic stimulation For personal use only. (TMS) were included. The following databases were used to conduct the search: MEDLINE/ PubMed, PsycINFO, PsycArticles, and Cochrane (Search Trials, CENTRAL). -

ON-LINE FIG 1. Selected Images of the Caudal Midbrain (Upper Row
ON-LINE FIG 1. Selected images of the caudal midbrain (upper row) and middle pons (lower row) from 4 of 13 total postmortem brains illustrate excellent anatomic contrast reproducibility across individual datasets. Subtle variations are present. Note differences in the shape of cerebral peduncles (24), decussation of superior cerebellar peduncles (25), and spinothalamic tract (12) in the midbrain of subject D (top right). These can be attributed to individual anatomic variation, some mild distortion of the brain stem during procurement at postmortem examination, and/or differences in the axial imaging plane not easily discernable during its prescription parallel to the anterior/posterior commissure plane. The numbers in parentheses in the on-line legends refer to structures in the On-line Table. AJNR Am J Neuroradiol ●:●●2019 www.ajnr.org E1 ON-LINE FIG 3. Demonstration of the dentatorubrothalamic tract within the superior cerebellar peduncle (asterisk) and rostral brain stem. A, Axial caudal midbrain image angled 10° anterosuperior to posteroinferior relative to the ACPC plane demonstrates the tract traveling the midbrain to reach the decussation (25). B, Coronal oblique image that is perpendicular to the long axis of the hippocam- pus (structure not shown) at the level of the ventral superior cerebel- lar decussation shows a component of the dentatorubrothalamic tract arising from the cerebellar dentate nucleus (63), ascending via the superior cerebellar peduncle to the decussation (25), and then enveloping the contralateral red nucleus (3). C, Parasagittal image shows the relatively long anteroposterior dimension of this tract, which becomes less compact and distinct as it ascends toward the thalamus. ON-LINE FIG 2. -

Multistable Properties of Human Subthalamic Nucleus Neurons in Parkinson’S Disease
Multistable properties of human subthalamic nucleus neurons in Parkinson’s disease Jeremy W. Chopeka,1, Hans Hultbornb, and Robert M. Brownstonea,2 aDepartment of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, University College London, WC1N 3BG London, United Kingdom; and bDepartment of Neuroscience, University of Copenhagen, 2200 Copenhagen N, Denmark Edited by Peter L. Strick, University of Pittsburgh, Pittsburgh, PA, and approved October 15, 2019 (received for review July 18, 2019) To understand the function and dysfunction of neural circuits, it is thorough characterization of complex neuronal properties is necessary to understand the properties of the neurons participating critical for understanding the modus operandi of neural circuits. in the behavior, the connectivity between these neurons, and the The connectivity of the excitatory subthalamic nucleus (STN) neuromodulatory status of the circuits at the time they are producing of the basal ganglia is well understood: it receives inputs from the the behavior. Such knowledge of human neural circuits is difficult, globus pallidus externa (GPe), motor cortex, and substantia nigra at best, to obtain. Here, we study firing properties of human pars compacta, and projects to the GPe, globus pallidus interna, subthalamic neurons, using microelectrode recordings and microstim- and substantia nigra pars reticulata. Furthermore, the basic ulation during awake surgery for Parkinson’s disease. We dem- electrophysiological properties of these neurons is reasonably onstrate that low-amplitude, brief trains of microstimulation can lead well understood, with resurgent and persistent sodium- and to persistent changes in neuronal firing behavior including switching calcium-dependent potassium conductances playing key roles for between firing rates, entering silent periods, or firing several bursts repetitive firing, and low-threshold calcium currents playing a then entering a silent period. -

Neuroimaging Advances in Deep Brain Stimulation: Review of Indications, Anatomy, and Brain Connectomics
Published August 13, 2020 as 10.3174/ajnr.A6693 REVIEW ARTICLE Neuroimaging Advances in Deep Brain Stimulation: Review of Indications, Anatomy, and Brain Connectomics E.H. Middlebrooks, R.A. Domingo, T. Vivas-Buitrago, L. Okromelidze, T. Tsuboi, J.K. Wong, R.S. Eisinger, L. Almeida, M.R. Burns, A. Horn, R.J. Uitti, R.E. Wharen Jr, V.M. Holanda, and S.S. Grewal ABSTRACT SUMMARY: Deep brain stimulation is an established therapy for multiple brain disorders, with rapidly expanding potential indi- cations. Neuroimaging has advanced the field of deep brain stimulation through improvements in delineation of anatomy, and, more recently, application of brain connectomics. Older lesion-derived, localizationist theories of these conditions have evolved to newer, network-based “circuitopathies,” aided by the ability to directly assess these brain circuits in vivo through the use of advanced neuroimaging techniques, such as diffusion tractography and fMRI. In this review, we use a combination of ultra-high-field MR imaging and diffusion tractography to highlight relevant anatomy for the currently approved indications for deep brain stimulation in the United States: essential tremor, Parkinson disease, drug-resistant epilepsy, dystonia, and obsessive-compulsive disorder. We also review the literature regarding the use of fMRI and diffusion tractography in under- standing the role of deep brain stimulation in these disorders, as well as their potential use in both surgical targeting and de- vice programming. ABBREVIATIONS: AL ¼ ansa lenticularis; ALIC -

Dorsal “Thalamus”
Dorsal “Thalamus” Medical Neuroscience Dr. Wiegand The Diencephalon The Diencephalon InterthalamicInterthalamic adhesionadhesion ThalamusThalamus EpithalamusEpithalamus HypothalamusHypothalamus (Pineal(Pineal && Habenula)Habenula) PituitaryPituitary SubthalamusSubthalamus 1 The “Dorsal” Thalamus | Sensory integration nucleus – gateway to the cerebral cortex | Afferents from both rostral and caudal central nervous system structures | Efferents primarily to cerebral cortex via four principal “radiations” | Associated with motor, sensory, limbic and vegetative functions External medullary lamina Anterior n. 3rd Internal capsule Ventricle Medial n. Medial Lateral n. Internal capsule * Reticular n. Internal * Interthalamic adhesion medullary lamina 2 General Organization medialmedial nucleinuclei anterioranterior nuclei nuclei internalinternal medullarymedullary laminalamina laterallateral nuclei nuclei dorsaldorsal tiertier pulvinarpulvinar geniculategeniculate ventralventral tiertier bodiesbodies Frontal Section intralaminarintralaminar nucleinuclei reticularreticular nuclei nuclei 3rd Ventricle externalexternalexternalexternal medullarymedullary laminalamina internalinternal laminalamina medullarymedullary laminalamina 3 Thalamic Nuclei | Anterior | Lateral z Dorsal Tier • lateral dorsal • lateral posterior • pulvinar z Ventral Tier • ventral anterior • ventral lateral • ventral posterior (VLP & VPM) • posterior nucleus Thalamic Nuclei | Medial z medial/medial dorsal z midline nuclei | Pulvinar | Geniculate bodies | Reticular | Intralaminar -

Motor Systems Basal Ganglia
Motor systems 409 Basal Ganglia You have just read about the different motor-related cortical areas. Premotor areas are involved in planning, while MI is involved in execution. What you don’t know is that the cortical areas involved in movement control need “help” from other brain circuits in order to smoothly orchestrate motor behaviors. One of these circuits involves a group of structures deep in the brain called the basal ganglia. While their exact motor function is still debated, the basal ganglia clearly regulate movement. Without information from the basal ganglia, the cortex is unable to properly direct motor control, and the deficits seen in Parkinson’s and Huntington’s disease and related movement disorders become apparent. Let’s start with the anatomy of the basal ganglia. The important “players” are identified in the adjacent figure. The caudate and putamen have similar functions, and we will consider them as one in this discussion. Together the caudate and putamen are called the neostriatum or simply striatum. All input to the basal ganglia circuit comes via the striatum. This input comes mainly from motor cortical areas. Notice that the caudate (L. tail) appears twice in many frontal brain sections. This is because the caudate curves around with the lateral ventricle. The head of the caudate is most anterior. It gives rise to a body whose “tail” extends with the ventricle into the temporal lobe (the “ball” at the end of the tail is the amygdala, whose limbic functions you will learn about later). Medial to the putamen is the globus pallidus (GP).