Shellfish Diseases and Their Management in Commercial Recirculating Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Guide to Culturing Parasites, Establishing Infections and Assessing Immune Responses in the Three-Spined Stickleback
ARTICLE IN PRESS Hook, Line and Infection: A Guide to Culturing Parasites, Establishing Infections and Assessing Immune Responses in the Three-Spined Stickleback Alexander Stewart*, Joseph Jacksonx, Iain Barber{, Christophe Eizaguirrejj, Rachel Paterson*, Pieter van West#, Chris Williams** and Joanne Cable*,1 *Cardiff University, Cardiff, United Kingdom x University of Salford, Salford, United Kingdom { University of Leicester, Leicester, United Kingdom jj Queen Mary University of London, London, United Kingdom #Institute of Medical Sciences, Aberdeen, United Kingdom **National Fisheries Service, Cambridgeshire, United Kingdom 1Corresponding author: E-mail: [email protected] Contents 1. Introduction 3 2. Stickleback Husbandry 7 2.1 Ethics 7 2.2 Collection 7 2.3 Maintenance 9 2.4 Breeding sticklebacks in vivo and in vitro 10 2.5 Hatchery 15 3. Common Stickleback Parasite Cultures 16 3.1 Argulus foliaceus 17 3.1.1 Introduction 17 3.1.2 Source, culture and infection 18 3.1.3 Immunology 22 3.2 Camallanus lacustris 22 3.2.1 Introduction 22 3.2.2 Source, culture and infection 23 3.2.3 Immunology 25 3.3 Diplostomum Species 26 3.3.1 Introduction 26 3.3.2 Source, culture and infection 27 3.3.3 Immunology 28 Advances in Parasitology, Volume 98 ISSN 0065-308X © 2017 Elsevier Ltd. http://dx.doi.org/10.1016/bs.apar.2017.07.001 All rights reserved. 1 j ARTICLE IN PRESS 2 Alexander Stewart et al. 3.4 Glugea anomala 30 3.4.1 Introduction 30 3.4.2 Source, culture and infection 30 3.4.3 Immunology 31 3.5 Gyrodactylus Species 31 3.5.1 Introduction 31 3.5.2 Source, culture and infection 32 3.5.3 Immunology 34 3.6 Saprolegnia parasitica 35 3.6.1 Introduction 35 3.6.2 Source, culture and infection 36 3.6.3 Immunology 37 3.7 Schistocephalus solidus 38 3.7.1 Introduction 38 3.7.2 Source, culture and infection 39 3.7.3 Immunology 43 4. -

ABSTRACT LUCKENBACH, JOHN ADAM. Breeding Biotechnology
ABSTRACT LUCKENBACH, JOHN ADAM. Breeding Biotechnology, Sex Determination, and Growth in Southern flounder, Paralichthys lethostigma. (Under the direction of Dr. John Godwin and Dr. Russell J. Borski). Southern flounder (Paralichthys lethostigma) support valuable, but declining US fisheries. This species is therefore a strong candidate for aquaculture to mitigate fishing impacts and stabilize seafood supply. Because female flounder reach substantially larger sizes than males, all-female culture is desirable for commercial aquaculture. Hence, a thorough understanding of sexual development, its timing and regulation by temperature is essential for optimization of flounder aquaculture. To better understand ovarian and testicular development in southern flounder, structural and cellular sex-distinguishing markers were studied using histological methods. We found that histologically discernible sex differentiation occurs in southern flounder at ~75-120 mm TL and that early differentiation is considerably delayed relative to its Japanese congener, P. olivaceus. High (28°C) and low (18°C) water temperatures, produced a higher proportion of males (96% and 78% males, respectively). The sex ratio at a mid-range (23°C) temperature was not different from 1:1. This suggests that southern flounder possess a temperature sensitive mechanism of sex determination. Growth was also affected by temperature with the temperature that maximized females inducing better growth. Aromatase cytochrome P450 (P450arom) is responsible for estrogen biosynthesis and plays a critical role in ovarian differentiation. We cloned ovarian P450arom and developed a qRT-PCR for assessment of early sex differentiation. The deduced amino acid sequence for southern flounder P450arom is very similar to P450arom in other teleosts. Comparison of P450arom intron sequences of southern flounder within and between different populations revealed substantial inter-individual variation that may affect sex determination responses. -

Fish and Fishery Products Hazards and Controls Guidance Fourth Edition – APRIL 2011
SGR 129 Fish and Fishery Products Hazards and Controls Guidance Fourth Edition – APRIL 2011 DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTRATION CENTER FOR FOOD SAFETY AND APPLIED NUTRITION OFFICE OF FOOD SAFETY Fish and Fishery Products Hazards and Controls Guidance Fourth Edition – April 2011 Additional copies may be purchased from: Florida Sea Grant IFAS - Extension Bookstore University of Florida P.O. Box 110011 Gainesville, FL 32611-0011 (800) 226-1764 Or www.ifasbooks.com Or you may download a copy from: http://www.fda.gov/FoodGuidances You may submit electronic or written comments regarding this guidance at any time. Submit electronic comments to http://www.regulations. gov. Submit written comments to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register. U.S. Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition (240) 402-2300 April 2011 Table of Contents: Fish and Fishery Products Hazards and Controls Guidance • Guidance for the Industry: Fish and Fishery Products Hazards and Controls Guidance ................................ 1 • CHAPTER 1: General Information .......................................................................................................19 • CHAPTER 2: Conducting a Hazard Analysis and Developing a HACCP Plan -
Schedule Onlinepdf
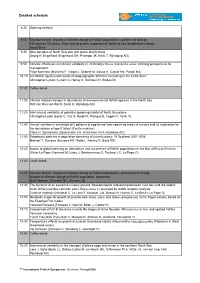
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

Viral Haemorrhagic Septicaemia Virus (VHSV): on the Search for Determinants Important for Virulence in Rainbow Trout Oncorhynchus Mykiss
Downloaded from orbit.dtu.dk on: Nov 08, 2017 Viral haemorrhagic septicaemia virus (VHSV): on the search for determinants important for virulence in rainbow trout oncorhynchus mykiss Olesen, Niels Jørgen; Skall, H. F.; Kurita, J.; Mori, K.; Ito, T. Published in: 17th International Conference on Diseases of Fish And Shellfish Publication date: 2015 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Olesen, N. J., Skall, H. F., Kurita, J., Mori, K., & Ito, T. (2015). Viral haemorrhagic septicaemia virus (VHSV): on the search for determinants important for virulence in rainbow trout oncorhynchus mykiss. In 17th International Conference on Diseases of Fish And Shellfish: Abstract book (pp. 147-147). [O-139] Las Palmas: European Association of Fish Pathologists. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. DISCLAIMER: The organizer takes no responsibility for any of the content stated in the abstracts. -

Disease of Aquatic Organisms 85:187
Vol. 85: 187–192, 2009 DISEASES OF AQUATIC ORGANISMS Published July 23 doi: 10.3354/dao02073 Dis Aquat Org Enhanced mortality in Nile tilapia Oreochromis niloticus following coinfections with ichthyophthiriasis and streptococcosis De-Hai Xu*, Craig A. Shoemaker, Phillip H. Klesius US Department of Agriculture, Agricultural Research Service, Aquatic Animal Health Research Laboratory, 990 Wire Road, Auburn, Alabama 36832, USA ABSTRACT: Ichthyophthirius multifiliis Fouquet (Ich) and Streptococcus iniae are 2 major pathogens of cultured Nile tilapia Oreochromis niloticus (L). Currently there is no information available for the effect of coinfection by Ich and S. iniae on fish. The objective of this study was to determine the effects of parasite load and Ich development size on fish mortality following S. iniae infection. Low mortality (≤20%) was observed in tilapia exposed to Ich or S. iniae alone. Mortalities increased from 38% in tilapia exposed to Ich at 10 000 theronts fish–1 to 88% in fish at 20 000 theronts fish–1 follow- ing S. iniae exposure. The median days to death were significantly fewer (7 d) in fish exposed to Ich at 20 000 theronts fish–1 than fish exposed to 10 000 theronts fish–1 (10 d). A positive correlation (cor- relation coefficient = 0.83) was noted between tilapia mortality and size of Ich trophonts at the time of S. iniae challenge. Fish parasitized with well-developed trophonts (Day 4, 2 × 107 µm3 in volume) suffered higher mortality (47.5%) than fish (10.0%) infested by young trophonts (Hour 4, 1.3 × 104 µm3 in volume) after S. iniae challenge. -

New Zealand's Genetic Diversity
1.13 NEW ZEALAND’S GENETIC DIVERSITY NEW ZEALAND’S GENETIC DIVERSITY Dennis P. Gordon National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6022, New Zealand ABSTRACT: The known genetic diversity represented by the New Zealand biota is reviewed and summarised, largely based on a recently published New Zealand inventory of biodiversity. All kingdoms and eukaryote phyla are covered, updated to refl ect the latest phylogenetic view of Eukaryota. The total known biota comprises a nominal 57 406 species (c. 48 640 described). Subtraction of the 4889 naturalised-alien species gives a biota of 52 517 native species. A minimum (the status of a number of the unnamed species is uncertain) of 27 380 (52%) of these species are endemic (cf. 26% for Fungi, 38% for all marine species, 46% for marine Animalia, 68% for all Animalia, 78% for vascular plants and 91% for terrestrial Animalia). In passing, examples are given both of the roles of the major taxa in providing ecosystem services and of the use of genetic resources in the New Zealand economy. Key words: Animalia, Chromista, freshwater, Fungi, genetic diversity, marine, New Zealand, Prokaryota, Protozoa, terrestrial. INTRODUCTION Article 10b of the CBD calls for signatories to ‘Adopt The original brief for this chapter was to review New Zealand’s measures relating to the use of biological resources [i.e. genetic genetic resources. The OECD defi nition of genetic resources resources] to avoid or minimize adverse impacts on biological is ‘genetic material of plants, animals or micro-organisms of diversity [e.g. genetic diversity]’ (my parentheses). -

FIELD GUIDE to WARMWATER FISH DISEASES in CENTRAL and EASTERN EUROPE, the CAUCASUS and CENTRAL ASIA Cover Photographs: Courtesy of Kálmán Molnár and Csaba Székely
SEC/C1182 (En) FAO Fisheries and Aquaculture Circular I SSN 2070-6065 FIELD GUIDE TO WARMWATER FISH DISEASES IN CENTRAL AND EASTERN EUROPE, THE CAUCASUS AND CENTRAL ASIA Cover photographs: Courtesy of Kálmán Molnár and Csaba Székely. FAO Fisheries and Aquaculture Circular No. 1182 SEC/C1182 (En) FIELD GUIDE TO WARMWATER FISH DISEASES IN CENTRAL AND EASTERN EUROPE, THE CAUCASUS AND CENTRAL ASIA By Kálmán Molnár1, Csaba Székely1 and Mária Láng2 1Institute for Veterinary Medical Research, Centre for Agricultural Research, Hungarian Academy of Sciences, Budapest, Hungary 2 National Food Chain Safety Office – Veterinary Diagnostic Directorate, Budapest, Hungary FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Ankara, 2019 Required citation: Molnár, K., Székely, C. and Láng, M. 2019. Field guide to the control of warmwater fish diseases in Central and Eastern Europe, the Caucasus and Central Asia. FAO Fisheries and Aquaculture Circular No.1182. Ankara, FAO. 124 pp. Licence: CC BY-NC-SA 3.0 IGO The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. -

Molecular Interaction Between Fish Pathogens and Host Aquatic Animals
K. Tsukamoto, T. Kawamura, T. Takeuchi, T. D. Beard, Jr. and M. J. Kaiser, eds. Fisheries for Global Welfare and Environment, 5th World Fisheries Congress 2008, pp. 277–288. © by TERRAPUB 2008. Molecular Interaction between Fish Pathogens and Host Aquatic Animals Laura L. Brown* and Stewart C. Johnson National Research Council of Canada Institute for Marine Biosciences 1411 Oxford Street Halifax, NS, B3H 3Z1, Canada Present address: Fisheries and Oceans Canada Pacific Biological Station 3190 Hammond Bay Road Nanaimo, NS, V9T 6N7, Canada *E-mail: [email protected] We have studied the host-pathogen interactions between Atlantic salmon (Salmo salar L.) and Aeromonas salmonicida. Sequencing the genome of the bacterium allowed us to investigate virulence factors and other gene products with potential as vaccines. Using knock-out mutants of A. salmonicida, we identified key virulence factors. Proteomics studies of bacterial cells grown in a variety of media as well as in an in vivo implant system revealed differential protein production and have shed new light on bacterial proteins such as superoxide dismutase, pili and flagellar proteins, type three secretion systems, and their roles in A. salmonicida pathogenicity. We constructed a whole ge- nome DNA microarray to use in comparative genomic hybridizations (M-CGH) and bacterial gene expression studies. Carbohydrate analysis has shown the variation in LPS between strains and reveals the importance of LPS in viru- lence. Salmon were challenged with A. salmonicida and tissues were taken to construct suppressive subtractive hybridization libraries to investigate differ- ential host gene expression. We constructed an Atlantic salmon cDNA microarray to investigate the host response to A. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0037370 A1 Corbeil Et Al
US 2015 0037370A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0037370 A1 Corbeil et al. (43) Pub. Date: Feb. 5, 2015 (54) DIATOM-BASEDVACCINES (86). PCT No.: PCT/US2O12/062112 S371 (c)(1), (71) Applicants: The Regents of the University of (2) Date: Apr. 23, 2014 California, Oakland, CA (US); Synaptic Related U.S. Application Data Research, LLC, Baltimore, MD (US) (60) Provisional application No. 61/553,139, filed on Oct. (72) Inventors: Lynette B. Corbeil, San Diego, CA 28, 2011. (US); Mark Hildebrand, La Jolla, CA Publication Classification (US); Roshan Shrestha, San Diego, CA (US); Aubrey Davis, Lakeside, CA (51) Eiko.29s (2006.01) (US) Rachel Schrier, Del Mar, CA CI2N 7/00 (2006.01) (US); George A. Oyler, Lincoln, NE A6139/02 (2006.01) (US); Julian N. Rosenberg, Naugatuck, A61E36/06 (2006.01) CT (US) A6139/02 (2006.01) (52) U.S. Cl. (73) Assignees: SYNAPTIC RESEARCH, LLC, CPC ............... A61K 39/295 (2013.01); A61K 36/06 Baltimore, MD (US): THE REGENTS (2013.01); A61 K39/107 (2013.01); A61 K OF THE UNIVERSITY OF 39/102 (2013.01); C12N 700 (2013.01); A61 K CALIFORNIA, Oakland, CA (US) 2039/523 (2013.01) USPC .................. 424/2011; 424/93.21; 424/261.1; y x- - - 9 (57) ABSTRACT 22) PCT Fled: Oct. 26, 2012 This invention pprovides diatom-based vaccines. Patent Application Publication Feb. 5, 2015 Sheet 1 of 19 US 2015/0037370 A1 83 : RE: Repests 388x ExF8. Patent Application Publication Feb. 5, 2015 Sheet 2 of 19 US 2015/0037370 A1 Fig. -

Notophthalmus Viridescens) by a New Species of Amphibiocystidium, a Genus of Fungus-Like Mesomycetozoan Parasites Not Previously Reported in North America
203 Widespread infection of the Eastern red-spotted newt (Notophthalmus viridescens) by a new species of Amphibiocystidium, a genus of fungus-like mesomycetozoan parasites not previously reported in North America T. R. RAFFEL1,2*, T. BOMMARITO 3, D. S. BARRY4, S. M. WITIAK5 and L. A. SHACKELTON1 1 Center for Infectious Disease Dynamics, Biology Department, Penn State University, University Park, PA 16802, USA 2 Department of Biology, University of South Florida, Tampa, FL 33620, USA 3 Cooperative Wildlife Research Lab, Department of Zoology, Southern Illinois University, Carbondale, IL 62901, USA 4 Department of Biological Sciences, Marshall University, Huntington, WV 25755, USA 5 Department of Plant Pathology, Penn State University, University Park, PA 16802, USA (Received 21 March 2007; revised 17 August 2007; accepted 20 August 2007; first published online 12 October 2007) SUMMARY Given the worldwide decline of amphibian populations due to emerging infectious diseases, it is imperative that we identify and address the causative agents. Many of the pathogens recently implicated in amphibian mortality and morbidity have been fungal or members of a poorly understood group of fungus-like protists, the mesomycetozoans. One mesomycetozoan, Amphibiocystidium ranae, is known to infect several European amphibian species and was associated with a recent decline of frogs in Italy. Here we present the first report of an Amphibiocystidium sp. in a North American amphibian, the Eastern red-spotted newt (Notophthalmus viridescens), and characterize it as the new species A. viridescens in the order Dermocystida based on morphological, geographical and phylogenetic evidence. We also describe the widespread and seasonal distribution of this parasite in red-spotted newt populations and provide evidence of mortality due to infection. -

Chemical Pollution of the Oceans Toxic Chemical Pollutants in the Oceans Have Been Shown Capable of Causing a Wide Range of Human Diseases
Supplementary Appendix to “Human Health and Ocean Pollution”, Annals of Global Health, 2020 This Supplementary Appendix contains additional references and documentation supporting the information presented in the report, Human Health and Ocean Pollution. Chemical Pollution of the Oceans Toxic chemical pollutants in the oceans have been shown capable of causing a wide range of human diseases. Toxicological and epidemiological studies document that pollutants such as toxic metals, POPs, dioxins, plastics chemicals, and pesticides can cause cardiovascular effects, developmental and neurobehavioral disorders, metabolic disease, endocrine disruption and cancer. Table 1 in this Supplementary Appendix summarizes the known links between chemical pollutants in the oceans and a range of human health outcomes. The strengths of the associations listed in Table 1 vary depending on the nature of the studies establishing these associations. Some associations have been assessed in systematic reviews and meta-analyses of animal and human data.1 2 Some are single cross-sectional or case-control studies. There are now a growing number of relevant epidemiological studies, including powerful prospective cohort studies, such as the Nurses’ Health Study II and the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS)3 Findings from these investigations are strengthening the evidence base for associations between exposures to organic chemical pollutants and adverse health outcomes. Supplementary Appendix Table 1. Adverse Human Health Outcomes