Soil Salinity in Agricultural Systems: the Basics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Irrigation Management with Saline Water
IRRIGATION MANAGEMENT WITH SALINE WATER Dana O. Porter, P.E. Thomas Marek, P.E. Associate Professor and Extension Senior Research Engineer & Agricultural Engineering Specialist Superintendent, North Research Field, Texas Cooperative Extension and Etter Texas Agricultural Experiment Station Texas Agricultural Experiment Station Texas A&M University Agricultural Texas A&M University Agricultural Research and Extension Center Research and Extension Center 1102 E. FM 1294 6500 Amarillo Blvd W. Lubbock, Texas 79403 Amarillo, TX 79106 Voice: 806-746-6101 Voice: (806) 677-5600 Fax: 806-746-4057 Fax: (806) 677-5644 E-mail: [email protected] E-mail: [email protected] INTRODUCTION One of the most common water quality concerns for irrigated agriculture is salinity. Recommendations for effective management of irrigation water salinity depend upon local soil properties, climate, and water quality; options of crops and rotations; and irrigation and farm management capabilities. What Is Salinity? All major irrigation water sources contain dissolved salts. These salts include a variety of natural occurring dissolved minerals, which can vary with location, time, and water source. Many of these mineral salts are micronutrients, having beneficial effects. However, excessive total salt concentration or excessive levels of some potentially toxic elements can have detrimental effects on plant health and/or soil conditions. The term “salinity” is used to describe the concentration of (ionic) salt species, generally including: calcium (Ca2+ ), magnesium (Mg2+ ), sodium (Na+ ), potassium + - - 2- 2- (K ), chloride (Cl ), bicarbonate (HCO3 ), carbonate(CO3 ), sulfate (SO4 ) and others. Salinity is expressed in terms of electrical conductivity (EC), in units of millimhos per centimeter (mmhos/cm), micromhos per centimeter (µmhos/cm), or deciSiemens per meter (dS/m). -

Salinity Distribution and Variation with Freshwater Inflow and Tide, And
Salinity Distribution and Variation with Freshwater Inflow and Tide, and Potential Changes in Salinity due to Altered Freshwater Inflow in the Charlotte Harbor Estuarine System, Florida By Yvonne E. Stoker U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 92-4062 Prepared in cooperation with the FLORIDA DEPARTMENT OF ENVIRONMENTAL REGULATION Tallahassee, Florida 1992 U.S. DEPARTMENT OF THE INTERIOR MANUEL LUJAN, JR., Secretary U.S. GEOLOGICAL SURVEY DALLAS L. PECK, Director For additional information, Copies of this report may be write to: purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Books and Open-File Reports Section Suite 3015 Federal Center 227 North Bronough Street Box 25425 Tallahassee, Florida 32301 Denver, Colorado 80225 CONTENTS Abstract 1 Introduction 1 Purpose and scope 3 Previous studies 4 Acknowledgments 4 Description of the study area and factors affecting salinity variation Freshwater inflow 4 Tide 7 Water density 8 Study methods 8 Salinity distribution in Charlotte Harbor 9 Salinity variations with freshwater inflow and tide 13 Variations with freshwater inflow 14 Tidal Caloosahatchee River 14 Upper Charlotte Harbor 17 Lower Charlotte Harbor 23 Variations with tide 23 Potential salinity changes due to altered freshwater inflow 24 Summary and conclusions 28 Selected references 29 Figure 1. Map showing study area and drainage basins 2 2. Map showing Charlotte Harbor and subarea boundaries 3 3. Map showing depth of the Charlotte Harbor estuarine system 5 4. Graphs showing daily mean discharge and monthly rainfall in the Peace, Myakka, and Caloosahatchee River basins, June 1982 to May 1987 6 5. Sketch showing generalization of highly stratified, partially mixed, and well-mixed salinity patterns in an estuary 8 6. -

Effects of Saline Water and Exogenous Application of Hydrogen Peroxide (H2O2) on Soursop (Annona Muricata L.) at Vegetative Stage
AJCS 13(03):472-479 (2019) ISSN:1835-2707 doi: 10.21475/ajcs.19.13.03.p1583 Effects of saline water and exogenous application of hydrogen peroxide (H2O2) on Soursop (Annona muricata L.) at vegetative stage Luana Lucas de Sá Almeida Veloso1, Carlos Alberto Vieira de Azevedo1, André Alisson Rodrigues da Silva1, Geovani Soares de Lima1*, Hans Raj Gheyi2, Raul Araújo da Nóbrega1, Francisco Wesley Alves Pinheiro1, Rômulo Carantino Moreira Lucena1 1Federal University of Campina Grande, Academic Unit of Agricultural Engineering, Campina Grande, 58.109-970, Paraíba, Brazil 2Federal University of Recôncavo of Bahia, Nucleus of Soil and Water Engineering, Cruz das Almas, 44.380-000, Bahia, Brazil *Corresponding author: [email protected] Abstract Soursop is a fruit of great socioeconomic importance for the northeastern region of Brazil. However, the quantitative and qualitative limitation of the water resources of this region has reduced its production. The objective of this study was to evaluate the growth of ‘Morada Nova’ soursop plants irrigated with saline water and subjected to exogenous application of hydrogen peroxide through seed immersion and foliar spray. The study was conducted in plastic pots adapted as lysimeters, using a eutrophic Regolithic Neosol with sandy loam texture under greenhouse conditions. Treatments were distributed in randomized blocks, in a 4 x 4 factorial arrangement, corresponding to four levels of irrigation water electrical conductivity – ECw (0.7; 1.7; 2.7 and 3.7 dS m-1) and four concentrations of hydrogen peroxide – H2O2 (0, 25, 50 and 75 µM), with three replicates and one plant per plot. Foliar applications of H2O2 began 15 days after transplanting (DAT) and were carried out every 15 days at 17:00 h, after the sunset, by manually spraying the H2O2 solutions with a sprayer in such a way to completely wet the leaves (spraying the abaxial and adaxial faces). -

DESALINATION: Balancing the Socioeconomic Benefits and Environmental Costs
DESALINATION: Balancing the Socioeconomic Benefits and Environmental Costs www.research.natixis.com https://gsh.cib.natixis.com executive summary Chapter 1 Making sense of desalination: technological, financial and economic aspects of desalination assets Chapter 2 Sustainability assessment of desalination assets: recognizing the socioeconomic benefits and mitigating environmental costs of desalination Chapter 3 Desalination sustainability performance scorecard acknowledgements appendix biblioghraphy TABLE OF CONTENTS OF TABLE 1. Making sense of desalination: technological, financial and economic aspects of desalination assets 1. DESALINATION TECHNOLOGIES 2. FINANCIAL AND ECONOMIC ASPECTS OF DESALINATION ASSETS 1.1. AN OVERVIEW OF DESALINATION TECHNOLOGIES 2.1.THE DEVELOPMENT AND FINANCING OF DESALINATION ASSETS Thermal desalination: Multistage Flash Distillation and Multieffect Distillation Building and operating desalination assets: complex and evolving value chain Membrane desalination: Reverse Osmosis Project development models: fine-tuning Hybridization of thermal and the appropriate risk-sharing model membrane desalination Bringing capital to desalination assets: A set of parameters to assess the performance an increasingly strategic issue and efficiency of desalination assets Case study of desalination in Israel: innovative 1.2. A BRIEF HISTORY AND financing schemes achieving some of the GEOGRAPHICAL DISTRIBUTION OF lowest desalinated water costs worldwide DESALINATION TECHNOLOGIES Case study of desalination in Singapore: The market -

Soil Salinity Type Effects on the Relationship Betweenthe Electrical
sustainability Article Soil Salinity Type Effects on the Relationship between the Electrical Conductivity and Salt Content for 1:5 Soil-to-Water Extract Amin I. Ismayilov 1, Amrakh I. Mamedov 2,* , Haruyuki Fujimaki 2 , Atsushi Tsunekawa 2 and Guy J. Levy 3 1 Institute of Soil Science and Agrichemistry, Azerbaijan National Academy of Sciences (ANAS), Baku AZ1073, Azerbaijan; [email protected] 2 Arid Land Research Center, Tottori University, Tottori 680-0001, Japan; [email protected] (H.F.); [email protected] (A.T.) 3 Institute of Soil, Water and Environmental Sciences, ARO, Rishon LeZion 7505101, Israel; [email protected] * Correspondence: [email protected] Abstract: Soil salinity severely affects soil ecosystem quality and crop production in semi-arid and arid regions. A vast quantity of data on soil salinity has been collected by research organizations of the Commonwealth of Independent States (CIS, formerly USSR) and many other countries over the last 70 years, but using them in the current international network (irrigation and reclamation strategy) is complicated. This is because in the CIS countries salinity was expressed by total soluble salts as a percentage on a dry-weight basis (total soluble salts, TSS, %) and eight salinity types − 2− − + (chemistry) determined by the ratios of the anions and cations (Cl , SO4 , HCO3 , and Na , Ca2+, Mg2+) in diluted soil water extract (soil/water = 1:5) without assessing electrical conductivity (EC). Measuring the EC (1:5) is more convenient, yet EC is not only affected by the concentration Citation: Ismayilov, A.I.; Mamedov, but also characteristics of the ions and the salinity chemistry. -

Status of Soil Salinity in California
it a major item in his joint presentation percent of construction, operation, and Although progress has been made, the to Congress and meeting with former maintenance costs. Basin states see the need for expanded President Nixon in 1972. In 1975, the Forum recommended wa- salinity control to maintain the numeric ter quality standards for salinity, includ- Proposed solutions criteria. Bills now before Congress would ing numeric criteria of 723 mg/L below authorize five additional salinity control The salinity problem has the potential to Hoover Dam, 747 mg/L below Parker units to be constructed by the Depart- cause lengthy legal and political battles Dam, and 879 mg/L at Imperial Dam. ment of the Interior, give the US. De- between the Upper and Lower Basin Their proposal also called for prompt partment of Agriculture specific author- states. The Lower Basin wants to pre- construction of the salinity control units ity for a program of on-farm Colorado vent salinity increases that would result authorized by P.L. 93-320, construction River salinity control measures in coop- from further upstream development; Up- of additional units upon completion of eration with local landowners, and pro- per Basin states are concerned that the planning reports, implementation of on- vide for 25 percent of the construction salinity issue could prevent future in- farm water management practices to costs to be paid by the Basin states. creases in their water use. control salinity, limitations on industrial In other efforts to control the river’s The states began to work together and municipal discharges, use of saline salinity, the Basin states have adopted a and with the federal government in the water for industrial purposes, and the policy calling for a no-salt return from late 1960s, and in the early 1970s several inclusion of the salinity components of industrial discharges and limiting the steps were taken to deal with the prob- water quality management plans devel- incremental increase permitted from lems. -
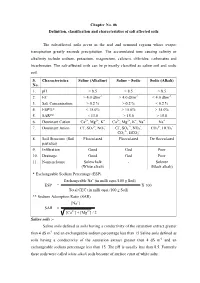
Chapter No. 06 Definition, Classification and Characteristics of Salt Affected Soils
Chapter No. 06 Definition, classification and characteristics of salt affected soils The salt-affected soils occur in the arid and semiarid regions where evapo- transpiration greatly exceeds precipitation. The accumulated ions causing salinity or alkalinity include sodium, potassium, magnesium, calcium, chlorides, carbonates and bicarbonates. The salt-affected soils can be primarily classified as saline soil and sodic soil. S. Characteristics Saline (Alkaline) Saline – Sodic Sodic (Alkali) No. 1. pH < 8.5 > 8.5 > 8.5 2. EC > 4.0 dSm-1 > 4.0 dSm-1 < 4.0 dSm-1 3. Salt Concentration > 0.2 % > 0.2 % < 0.2 % 4. ESP%* < 15.0% > 15.0% > 15.0% 5. SAR** < 13.0 > 15.0 > 15.0 6. Dominant Cation Ca2+, Mg2+, K+ Ca2+, Mg2+, K+, Na+ Na+ - 2- - - 2- - 2- - 7. Dominant Anion Cl , SO4 , NO3 Cl , SO4 , NO3 , CO3 , HCO3 2- - CO3 , HCO3 8. Soil Structure (Soil Flocculated Flocculated De flocculated particles) 9. Infiltration Good God Poor 10. Drainage Good God Poor 11. Nomenclature Solenchalk - Solentz (White alkali) (Black alkali) * Exchangeable Sodium Percentage (ESP) Exchangeable Na+ (in milli equi./100 g Soil) ESP = X 100 Total CEC (in milli equi./100 g Soil) ** Sodium Adsorption Ratio (SAR) [Na+] SAR = [Ca2+] + [Mg2+] / 2 Saline soils :- Saline soils defined as soils having a conductivity of the saturation extract greater than 4 dS m-1 and an exchangeable sodium percentage less than 15 Saline soils defined as soils having a conductivity of the saturation extract greater than 4 dS m-1 and an exchangeable sodium percentage less than 15. The pH is usually less than 8.5. -

Anguelova, M.D. and Huq, P., 2018. Effects of Salinity on Bubble Cloud
Journal of Marine Science and Engineering Article Effects of Salinity on Bubble Cloud Characteristics Magdalena D. Anguelova 1,* ID and Pablo Huq 2 1 Remote Sensing Division, Naval Research Laboratory, Washington, DC 20375, USA 2 College of Earth, Ocean, and Environment, University of Delaware, Newark, DE 19716, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-202-404-6342 Received: 13 November 2017; Accepted: 26 December 2017; Published: 29 December 2017 Abstract: A laboratory experiment investigates the influence of salinity on the characteristics of bubble clouds in varying saline solutions. Bubble clouds were generated with a water jet. Salinity, surface tension, and water temperature were monitored. Measured bubble cloud parameters include the number of bubbles, the void fraction, the penetration depth, and the cloud shape. The number of large (above 0.5 mm diameter) bubbles within a cloud increases by a factor of three from fresh to saline water of 20 psu (practical salinity units), and attains a maximum value for salinity of 12–25 psu. The void fraction also has maximum value in the range 12–25 psu. The results thus show that both the number of bubbles and the void fraction vary nonmonotonically with increasing salinity. The lateral shape of the bubble cloud does not change with increasing salinity; however, the lowest point of the cloud penetrates deeper as smaller bubbles are generated. Keywords: bubbles; bubble clouds; seawater salinity; surface tension; whitecaps; breaking waves; plunging water jet; air entrapment; gas exchange; energy dissipation 1. Introduction Bubble clouds arising from waves breaking at the ocean surface play an important role in the transport of momentum and scalars between the atmosphere and the ocean [1,2]. -

Managing Soil Salinity Tony Provin and J.L
E-60 3-12 Managing Soil Salinity Tony Provin and J.L. Pitt* f your soil has a high salinity content, the plants ing may cause salts to accumulate in both surface growing there will not be as vigorous as they would and underground waters. The surface runoff of these Ibe in normal soils. Seeds will germinate poorly, dissolved salts is what gives the salt content to our if at all, and the plants will grow slowly or become oceans and lakes. Fertilizers and organic amendments stunted. If the salinity concentration is high enough, also add salts to the soil. the plants will wilt and die, no matter how much you water them. Effects of salts on plants Routine soil testing can identify your soil’s salinity As soils become more saline, plants become unable levels and suggest measures you can take to correct to draw as much water from the soil. This is because the specific salinity problem in your soil. the plant roots contain varying concentrations of ions (salts) that create a natural flow of water from the soil Salinity and salt into the plant roots. The terms salt and salinity are often used inter- As the level of salinity in the soil nears that of the changeably, and sometimes incorrectly. A salt is sim- roots, however, water becomes less and less likely to ply an inorganic mineral that can dissolve in water. enter the root. In fact, when the soil salinity levels are Many people associate salt with sodium chloride— high enough, the water in the roots is pulled back into common table salt. -

Effects of Alternating Irrigation with Fresh and Saline Water on the Soil
water Article Effects of Alternating Irrigation with Fresh and Saline Water on the Soil Salt, Soil Nutrients, and Yield of Tomatoes Jingang Li 1 , Jing Chen 2,*, Zhongyi Qu 3,*, Shaoli Wang 4, Pingru He 5 and Na Zhang 6 1 College of Water Conservancy and Hydropower Engineering, Hohai University, Nanjing 210098, China 2 College of Agricultural Engineering, Hohai University, Nanjing 210098, China 3 Water Conservancy and Civil Engineering College, Inner Mongolia Agricultural University, Hohhot 010018, China 4 State Key Laboratory of Simulation and Regulation of Water Cycle in River Basin, Department of Irrigation and Drainage, China Institute of Water Resources and Hydropower Research, Beijing 100038, China 5 Key Laboratory of Agricultural Soil and Water Engineering in Arid and Semiarid Areas of Ministry of Education, Northwest A&F University, Yangling 712100, China 6 Ningxia Institute of Water Resources Research, Yinchuan 750021, China * Correspondence: [email protected] (J.C.); [email protected] (Z.Q.); Tel.: +86-139-1298-0055 (J.C.); +86-150-4910-9708 (Z.Q.) Received: 1 July 2019; Accepted: 13 August 2019; Published: 15 August 2019 Abstract: Saline water irrigation has become extremely important in arid and semi-arid areas in northwestern China. To study the effect of alternating irrigation models on the soil nutrients, soil salts, and yield of tomatoes with fresh water (total dissolved solids of 0.50 g L 1) and saline water · − (total dissolved solids of 3.01 g L 1), a two-year field experiment was carried out for tomatoes in the · -

Methods of Analysis Soil Sampling
Soil Sampling and Methods of Analysis Second Edition ß 2006 by Taylor & Francis Group, LLC. In physical science the first essential step in the direction of learning any subject is to find principles of numerical reckoning and practicable methods for measuring some quality connected with it. I often say that when you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot measure it, when you cannot express it in numbers, your knowledge is of a meagre and unsatisfactory kind; it may be the beginning of knowledge, but you have scarcely in your thoughts advanced to the state of science, whatever the matter may be. Lord Kelvin, Popular Lectures and Addresses (1891–1894), vol. 1, Electrical Units of Measurement Felix qui potuit rerum cognoscere causas. Happy the man who has been able to learn the causes of things. Virgil: Georgics (II, 490) ß 2006 by Taylor & Francis Group, LLC. Soil Sampling and Methods of Analysis Second Edition Edited by M.R. Carter E.G. Gregorich Canadian Society of Soil Science ß 2006 by Taylor & Francis Group, LLC. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2008 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-13: 978-0-8493-3586-0 (Hardcover) This book contains information obtained from authentic and highly regarded sources. -

Chapter 14, Salinity, Voluntary Estuary Monitoring Manual, March 2006
This document is Chapter 14 of the Volunteer Estuary Monitoring Manual, A Methods Manual, Second Edition, EPA-842-B-06-003. The full document be downloaded from: http://www.epa.gov/owow/estuaries/monitor/ Voluntary Estuary Monitoring Manual Chapter 14: Salinity March 2006 Chapter 1Salinity4 Because of its importance to estuarine ecosystems, salinity (the amount of dissolved salts in water) is commonly measured by volunteer monitoring programs. Photos (l to r): U.S. Environmental Protection Agency, R. Ohrel, The Ocean Conservancy, P. Bergstrom Unit Two: Physical Measures Chapter 14: Salinity Overview Because of its importance to estuarine ecosystems, salinity (the amount of dissolved salts in water) is commonly measured by volunteer monitoring programs. This chapter discusses the role of salinity in the estuarine environment and provides steps for measuring this water quality variable. 14-1 Volunteer Estuary Monitoring: A Methods Manual Chapter 14: Salinity Unit Two: Physical Measures About Salinity Salinity is simply a measure of the amount of mixing the two masses of water. The shape of salts dissolved in water. An estuary usually the estuary and the volume of river flow also exhibits a gradual change in salinity throughout influence this two-layer circulation. See its length, as fresh water entering the estuary Chapter 2 for more information. from tributaries mixes with seawater moving in from the ocean (Figure 14-1). Salinity is usually Role of Salinity in the Estuarine Ecosystem Salinity levels control, to a large degree, the Non-Tidal types of plants and animals that can live in Fresh Water different zones of the estuary. Freshwater Average (During species may be restricted to the upper reaches Annual Tidal Low Flow Salinity Tidal Limit Fresh Water Conditions) of the estuary, while marine species inhabit the < 0.5 ppt estuarine mouth.