Saline and Alkali Soils Are Soils That Saline Soil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Hardpan) for Precision Agriculture on So
SITE - SPECIFIC CHARACTERIZATION, MODELING AND SPATIAL ANALYSIS OF SUB-SOIL COMPACTION (HARDPAN) FOR PRECISION AGRICULTURE ON SOUTHEASTERN US SOILS by MEHARI ZEWDE TEKESTE (Under the Direction of Randy L. Raper and Ernest W. Tollner) ABSTRACT Natural and machinery traffic-induced subsoil compacted layers (soil hardpans) that are found in many southeastern US soils limit root growth with detrimental effects on crop productivity and the environment. Due to the spatial variability of hardpans, tillage management systems that use site-specific depth tillage applications may reduce fuel consumption compared to the conventional uniform depth tillage. The success of site-specific tillage or variable depth tillage depends on an accurate sensing of the hardpan layers, field positioning, and controlling the application of real-time or prescribed tillage. The over arching objective of the work was to understand and advance the art and science of soil compaction analysis and prediction with an eye towards compaction management in precision farming. The specific objectives were (1) To investigate the influences of soil parameters (soil moisture and bulk density), and the soil-cone frictional property on the interpretations of cone penetrometer data in predicting the magnitude and depth of hardpans, (2) To determine spatial variability for creating hardpan maps and (3) To investigate a passive acoustic based real-time soil compaction sensing method. The soil cone penetration problems were also modeled using finite element modeling to investigate the soil deformation patterns and evaluate the capability of the finite element method to predict the magnitude and depth of the hardpan. Laboratory experiments in a soil column study indicated that the soil cone penetration resistances were affected by soil moisture, bulk density and cone material type. -

HOW to IMPROVE SOIL DRAINAGE Charlotte Germane, Nevada County Master Gardener
GOT COMPACTION? HOW TO IMPROVE SOIL DRAINAGE Charlotte Germane, Nevada County Master Gardener From The Curious Gardener, Summer 2011 Do you suspect you might have a drainage problem in your garden? If your soil does not drain quickly enough, your plants will drown. Soils in the foothills Your soil drainage may not be as bad as you think it is. There’s so much talk in the foothills about clay soil that some gardeners assume they have poorly draining soil, and grumble about it, when they actually have pretty respectable loam. The USDA has mapped soil types and found that in the lower foothills the soil can be sandy loam over heavy sandy loam, or loam over clay loam. Above 2000 feet, it is typically loam over clay loam with cobblestones. An unusual feature of foothills soil is the serpentine outcropping. This combines poor drainage with toxic levels of magnesium. If you need to grow in a serpentine soil area, use raised beds. The serpentine soil under the beds will not provide adequate drainage. Another foothill soil issue that makes for poor drainage is “layered soil”. Soil naturally transitions from one kind to another, but layered soil means soil that changes abruptly, making it hard for water to move through easily. Layered soil occurs naturally (soil on top of rock or a clay pan) and can also be created by digging with rototillers and heavy equipment. Check for poor grading, over-irrigation, and thatched lawns Before you label your soil the culprit, walk your garden and evaluate the grading. It is possible that at the time of your home’s construction, or during a later landscaping project,the soil was graded so the water drained toward an area with no easy outlet. -

Agricultural Soil Compaction: Causes and Management
October 2010 Agdex 510-1 Agricultural Soil Compaction: Causes and Management oil compaction can be a serious and unnecessary soil aggregates, which has a negative affect on soil S form of soil degradation that can result in increased aggregate structure. soil erosion and decreased crop production. Soil compaction can have a number of negative effects on Compaction of soil is the compression of soil particles into soil quality and crop production including the following: a smaller volume, which reduces the size of pore space available for air and water. Most soils are composed of • causes soil pore spaces to become smaller about 50 per cent solids (sand, silt, clay and organic • reduces water infiltration rate into soil matter) and about 50 per cent pore spaces. • decreases the rate that water will penetrate into the soil root zone and subsoil • increases the potential for surface Compaction concerns water ponding, water runoff, surface soil waterlogging and soil erosion Soil compaction can impair water Soil compaction infiltration into soil, crop emergence, • reduces the ability of a soil to hold root penetration and crop nutrient and can be a serious water and air, which are necessary for water uptake, all of which result in form of soil plant root growth and function depressed crop yield. • reduces crop emergence as a result of soil crusting Human-induced compaction of degradation. • impedes root growth and limits the agricultural soil can be the result of using volume of soil explored by roots tillage equipment during soil cultivation or result from the heavy weight of field equipment. • limits soil exploration by roots and Compacted soils can also be the result of natural soil- decreases the ability of crops to take up nutrients and forming processes. -

Soils and Soil-Forming Material Technical Information Note 04 /2017 30Th November 2017
Soils and Soil-forming Material Technical Information Note 04 /2017 30th November 2017 Contents 1. Introduction to Soils ........................................................................................................................ 2 2. Components and Properties of Soil ................................................................................................ 7 3. Describing and Categorising soils .................................................................................................. 29 4. Policy, Regulation and Roles ......................................................................................................... 34 5. Soil Surveys, Handling and Management ..................................................................................... 40 6. Recommended Soil Specifications ................................................................................................ 42 7. References .................................................................................................................................... 52 “Upon this handful of soil our survival depends. Husband it and it will grow our food, our fuel, and our shelter and surround us with beauty. Abuse it and the soil will collapse and die, taking humanity with it.” From Vedas Sanskrit Scripture – circa 1500 BC The aim of this Technical Information Note is to assist Landscape Professionals (primarily landscape architects) when considering matters in relation to soils and soil-forming material. Soil is an essential requirement for providing -

Soil Salinity in Agricultural Systems: the Basics
Soil Salinity in Agricultural Systems: The Basics Jeffrey L. Ullman Agricultural & Biological Engineering University of Florida Strategies for Minimizing Salinity Problems and Optimizing Crop Production In-Service Training, Hastings, FL March 26, 2013 What is salt? What is Salt? . Salts are more than just sodium chloride (NaCl) . Salts consist of anions and cations . In terms of soil and irrigation water these generally include: Cations Anions Sodium Na+ Chlorides Cl- 2+ 2- Magnesium Mg Sulfates SO4 2+ 2- Calcium Ca Carbonates CO3 - Bicarbonates HCO3 What is Salt? . Other salts in agriculture + Potassium (K ) - Nitrate (NO3 ) Boron (B) • Often as boric acid (H3BO3, often written as B(OH)3) • Can form salts such as sodium borate (borax; Na2B4O7) Photo: Georgia Agriculture What is Salt? H O(l) NaCl(s) 2 Na+(aq) + Cl-(aq) (aq) indicates that Na+ and Cl- are hydrated ions Sodium sulfate Magnesium carbonate Source: Averill and Eldredge (2007) Types of Salts Some common salts NaCl Sodium chloride Table salt (halite) CO 2- 3 KCl Potassium chloride Muriate of potash Na+ 2- NaHCO3 Sodium bicarbonate Baking soda (nahcolite) SO4 - Cl CaSO4 Calcium sulfate Gypsum + K CaCO3 Calcium carbonate Calcite 2+ Ca MgSO Magnesium sulfate Epsom salt (epsomite) Mg2+ 4 K2SO4 Potassium sulfate Sulfate of potash (arcanite) HCO - 3 Glauber’s salt (thenardite Na SO Sodium sulfate 2 4 and mirabilite) Gypsum Calcite Thenardite Sources of Salt . Dissolution of parent rock material . Irrigation water . Saline groundwater . Fertilizers . Manure . Seawater intrusion Photo: J. Ullman Saline Soils . Accumulation of salts known as salination . Can occur in diverse types of soil with different physical, chemical and hydrologic properties Photo: USDA-NRCS Saline Soils . -

Soil Salinity Type Effects on the Relationship Betweenthe Electrical
sustainability Article Soil Salinity Type Effects on the Relationship between the Electrical Conductivity and Salt Content for 1:5 Soil-to-Water Extract Amin I. Ismayilov 1, Amrakh I. Mamedov 2,* , Haruyuki Fujimaki 2 , Atsushi Tsunekawa 2 and Guy J. Levy 3 1 Institute of Soil Science and Agrichemistry, Azerbaijan National Academy of Sciences (ANAS), Baku AZ1073, Azerbaijan; [email protected] 2 Arid Land Research Center, Tottori University, Tottori 680-0001, Japan; [email protected] (H.F.); [email protected] (A.T.) 3 Institute of Soil, Water and Environmental Sciences, ARO, Rishon LeZion 7505101, Israel; [email protected] * Correspondence: [email protected] Abstract: Soil salinity severely affects soil ecosystem quality and crop production in semi-arid and arid regions. A vast quantity of data on soil salinity has been collected by research organizations of the Commonwealth of Independent States (CIS, formerly USSR) and many other countries over the last 70 years, but using them in the current international network (irrigation and reclamation strategy) is complicated. This is because in the CIS countries salinity was expressed by total soluble salts as a percentage on a dry-weight basis (total soluble salts, TSS, %) and eight salinity types − 2− − + (chemistry) determined by the ratios of the anions and cations (Cl , SO4 , HCO3 , and Na , Ca2+, Mg2+) in diluted soil water extract (soil/water = 1:5) without assessing electrical conductivity (EC). Measuring the EC (1:5) is more convenient, yet EC is not only affected by the concentration Citation: Ismayilov, A.I.; Mamedov, but also characteristics of the ions and the salinity chemistry. -

Status of Soil Salinity in California
it a major item in his joint presentation percent of construction, operation, and Although progress has been made, the to Congress and meeting with former maintenance costs. Basin states see the need for expanded President Nixon in 1972. In 1975, the Forum recommended wa- salinity control to maintain the numeric ter quality standards for salinity, includ- Proposed solutions criteria. Bills now before Congress would ing numeric criteria of 723 mg/L below authorize five additional salinity control The salinity problem has the potential to Hoover Dam, 747 mg/L below Parker units to be constructed by the Depart- cause lengthy legal and political battles Dam, and 879 mg/L at Imperial Dam. ment of the Interior, give the US. De- between the Upper and Lower Basin Their proposal also called for prompt partment of Agriculture specific author- states. The Lower Basin wants to pre- construction of the salinity control units ity for a program of on-farm Colorado vent salinity increases that would result authorized by P.L. 93-320, construction River salinity control measures in coop- from further upstream development; Up- of additional units upon completion of eration with local landowners, and pro- per Basin states are concerned that the planning reports, implementation of on- vide for 25 percent of the construction salinity issue could prevent future in- farm water management practices to costs to be paid by the Basin states. creases in their water use. control salinity, limitations on industrial In other efforts to control the river’s The states began to work together and municipal discharges, use of saline salinity, the Basin states have adopted a and with the federal government in the water for industrial purposes, and the policy calling for a no-salt return from late 1960s, and in the early 1970s several inclusion of the salinity components of industrial discharges and limiting the steps were taken to deal with the prob- water quality management plans devel- incremental increase permitted from lems. -
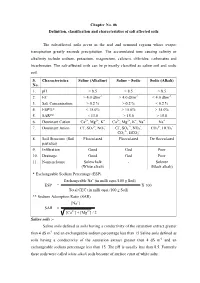
Chapter No. 06 Definition, Classification and Characteristics of Salt Affected Soils
Chapter No. 06 Definition, classification and characteristics of salt affected soils The salt-affected soils occur in the arid and semiarid regions where evapo- transpiration greatly exceeds precipitation. The accumulated ions causing salinity or alkalinity include sodium, potassium, magnesium, calcium, chlorides, carbonates and bicarbonates. The salt-affected soils can be primarily classified as saline soil and sodic soil. S. Characteristics Saline (Alkaline) Saline – Sodic Sodic (Alkali) No. 1. pH < 8.5 > 8.5 > 8.5 2. EC > 4.0 dSm-1 > 4.0 dSm-1 < 4.0 dSm-1 3. Salt Concentration > 0.2 % > 0.2 % < 0.2 % 4. ESP%* < 15.0% > 15.0% > 15.0% 5. SAR** < 13.0 > 15.0 > 15.0 6. Dominant Cation Ca2+, Mg2+, K+ Ca2+, Mg2+, K+, Na+ Na+ - 2- - - 2- - 2- - 7. Dominant Anion Cl , SO4 , NO3 Cl , SO4 , NO3 , CO3 , HCO3 2- - CO3 , HCO3 8. Soil Structure (Soil Flocculated Flocculated De flocculated particles) 9. Infiltration Good God Poor 10. Drainage Good God Poor 11. Nomenclature Solenchalk - Solentz (White alkali) (Black alkali) * Exchangeable Sodium Percentage (ESP) Exchangeable Na+ (in milli equi./100 g Soil) ESP = X 100 Total CEC (in milli equi./100 g Soil) ** Sodium Adsorption Ratio (SAR) [Na+] SAR = [Ca2+] + [Mg2+] / 2 Saline soils :- Saline soils defined as soils having a conductivity of the saturation extract greater than 4 dS m-1 and an exchangeable sodium percentage less than 15 Saline soils defined as soils having a conductivity of the saturation extract greater than 4 dS m-1 and an exchangeable sodium percentage less than 15. The pH is usually less than 8.5. -

Sustainable Soil Management
Top of Form ATTRAv2 page skip navigation 500 500 500 500 500 0 Search Bottom of Form 800-346-9140 Home | Site Map | Who We Are | Contact (English) Us | Calendar | Español | Text Only 800-411-3222 (Español) Home > Master Publication List > Sustainable Soil Management What Is Sustainable Soil Management Sustainable Agriculture? The printable PDF version of the Horticultural By Preston Sullivan entire document is available at: Crops NCAT Agriculture Specialist http://attra.ncat.org/attra- © NCAT 2004 pub/PDF/soilmgmt.pdf Field Crops ATTRA Publication #IP027/133 31 pages — 1.5 mb Download Acrobat Reader Soils & Compost Water Management Pest Management Organic Farming Livestock Marketing, Business & Risk Abstract Soybeans no-till planted into Management wheat stubble. This publication covers basic soil Photo by: Preston Sullivan Farm Energy properties and management steps toward building and maintaining healthy soils. Part I deals with basic Education soil principles and provides an understanding of living soils and how they work. In this section you will find answers to why soil organisms Other Resources and organic matter are important. Part II covers management steps to build soil quality on your farm. The last section looks at farmers who Master have successfully built up their soil. The publication concludes with a Publication List large resource section of other available information. Table of Contents Top of Form Part I. Characteristics of Sustainable Soils o Introduction o The Living Soil: Texture and Structure o The Living Soil: The Importance of Soil Organisms 1011223551022 o Organic Matter, Humus, and the Soil Foodweb o Soil Tilth and Organic Matter oi o Tillage, Organic Matter, and Plant Productivity o Fertilizer Amendments and Biologically Active Soils Go o Conventional Fertilizers Enter your o Top$oil—Your Farm'$ Capital email above o Summary of Part I and click Go. -

Nitrate Distribution in a Deep, Alluvial Unsaturated Zone: Geologic Control Vs
Nitrate Distribution in a Deep, Alluvial Unsaturated Zone: Geologic Control vs. Fertilizer Management Thomas Harter*, Katrin Heeren, William R. Horwath Department of Land, Air, and Water Resources University of California Davis, CA 95616 * corresponding author ph/530-752-2709; [email protected]; http://groundwater.ucdavis.edu Introduction For decades, nitrate leaching from agricultural sources (among others) has been a concern to agronomists, soil scientists, and hydrologists. Federal legislation first recognized the potential impacts to water resources in the early 1970s, when the Clean Water Act (CWA), the Safe Drinking Water Act (SDWA), the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), and other water pollution related legislation was enacted. Since then, countless efforts have been mounted by both the scientific-technical community and the agricultural industry to better understand the role of agricultural practices in determining the fate of fertilizer and pesticides in watersheds (including groundwater) and to improve agricultural management accordingly. From a groundwater perspective, much of the scientific work relating to nitrate has focused on two areas: documenting the extent of groundwater nitrate contamination; and investigating the fate of nitrogen in the soil root zone (including the potential for groundwater leaching) as it relates to particular agricultural crops and management practices. Rarely, these two research areas are linked within a single study and if they are, groundwater levels are typically close to the soil surface (less than 10 feet). In California’s valleys and basins, particularly in Central and Southern California, groundwater levels are frequently much deeper than 20 feet. The unsaturated zone between the land surface and the water table may therefore be from 20 to over 100 feet thick. -

Seed and Soil Dynamics in Shrubland Ecosystems: Proceedings; 2002 August 12–16; Laramie, WY
United States Department of Agriculture Seed and Soil Dynamics in Forest Service Rocky Mountain Shrubland Ecosystems: Research Station Proceedings Proceedings RMRS-P-31 February 2004 Abstract Hild, Ann L.; Shaw, Nancy L.; Meyer, Susan E.; Booth, D. Terrance; McArthur, E. Durant, comps. 2004. Seed and soil dynamics in shrubland ecosystems: proceedings; 2002 August 12–16; Laramie, WY. Proc. RMRS-P-31. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 216 p. The 38 papers in this proceedings are divided into six sections; the first includes an overview paper and documentation of the first Shrub Research Consortium Distinguished Service Award. The next four sections cluster papers on restoration and revegetation, soil and microsite requirements, germination and establishment of desired species, and community ecology of shrubland systems. The final section contains descriptions of the field trips to the High Plains Grassland Research Station and to the Snowy Range and Medicine Bow Peak. The proceedings unites many papers on germination of native seed with vegetation ecology, soil physio- chemical properties, and soil biology to create a volume describing the interactions of seeds and soils in arid and semiarid shrubland ecosystems. Keywords: wildland shrubs, seed, soil, restoration, rehabilitation, seed bank, seed germination, biological soil crusts Acknowledgments The symposium, field trips, and subsequent publication of this volume were made possible through the hard work of many people. We wish to thank everyone who took a part in ensuring the success of the meetings, trade show, and paper submissions. We thank the University of Wyoming Office of Academic Affairs, the Graduate School, and its Dean, Dr. -

Managing Soil Salinity Tony Provin and J.L
E-60 3-12 Managing Soil Salinity Tony Provin and J.L. Pitt* f your soil has a high salinity content, the plants ing may cause salts to accumulate in both surface growing there will not be as vigorous as they would and underground waters. The surface runoff of these Ibe in normal soils. Seeds will germinate poorly, dissolved salts is what gives the salt content to our if at all, and the plants will grow slowly or become oceans and lakes. Fertilizers and organic amendments stunted. If the salinity concentration is high enough, also add salts to the soil. the plants will wilt and die, no matter how much you water them. Effects of salts on plants Routine soil testing can identify your soil’s salinity As soils become more saline, plants become unable levels and suggest measures you can take to correct to draw as much water from the soil. This is because the specific salinity problem in your soil. the plant roots contain varying concentrations of ions (salts) that create a natural flow of water from the soil Salinity and salt into the plant roots. The terms salt and salinity are often used inter- As the level of salinity in the soil nears that of the changeably, and sometimes incorrectly. A salt is sim- roots, however, water becomes less and less likely to ply an inorganic mineral that can dissolve in water. enter the root. In fact, when the soil salinity levels are Many people associate salt with sodium chloride— high enough, the water in the roots is pulled back into common table salt.