1. General Information 2. Description 3. Details
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LCD for Diagnostic Pap Smear (L22842)
LCD for Diagnostic Pap Smear (L22842) All ICD-9 Codes (diagnosis codes) must be carried to their highest level of specification. ICD-9 Codes that Support Medical Necessity 054.10 GENITAL HERPES UNSPECIFIED 054.11 HERPETIC VULVOVAGINITIS 054.12 HERPETIC ULCERATION OF VULVA 078.0 MOLLUSCUM CONTAGIOSUM 078.10 VIRAL WARTS UNSPECIFIED 078.11 CONDYLOMA ACUMINATUM 078.19 OTHER SPECIFIED VIRAL WARTS 090.0 - EARLY CONGENITAL SYPHILIS SYMPTOMATIC - VENEREAL DISEASE 099.9 UNSPECIFIED 112.1 CANDIDIASIS OF VULVA AND VAGINA 112.2 CANDIDIASIS OF OTHER UROGENITAL SITES 171.6 MALIGNANT NEOPLASM OF CONNECTIVE AND OTHER SOFT TISSUE OF PELVIS 179 MALIGNANT NEOPLASM OF UTERUS-PART UNS 180.0 - MALIGNANT NEOPLASM OF ENDOCERVIX - MALIGNANT NEOPLASM OF 180.9 CERVIX UTERI UNSPECIFIED SITE 181 MALIGNANT NEOPLASM OF PLACENTA 182.0 - MALIGNANT NEOPLASM OF CORPUS UTERI EXCEPT ISTHMUS - 182.8 MALIGNANT NEOPLASM OF OTHER SPECIFIED SITES OF BODY OF UTERUS 183.0 - MALIGNANT NEOPLASM OF OVARY - MALIGNANT NEOPLASM OF 183.8 OTHER SPECIFIED SITES OF UTERINE ADNEXA 184.0 - MALIGNANT NEOPLASM OF VAGINA - MALIGNANT NEOPLASM OF 184.9 FEMALE GENITAL ORGAN SITE UNSPECIFIED 195.3 MALIGNANT NEOPLASM OF PELVIS 198.6 SECONDARY MALIGNANT NEOPLASM OF OVARY 198.82 SECONDARY MALIGNANT NEOPLASM OF GENITAL ORGANS 218.0 - SUBMUCOUS LEIOMYOMA OF UTERUS - LEIOMYOMA OF UTERUS 218.9 UNSPECIFIED 219.0 - BENIGN NEOPLASM OF CERVIX UTERI - BENIGN NEOPLASM OF 219.9 UTERUS PART UNSPECIFIED 220 BENIGN NEOPLASM OF OVARY 221.0 - BENIGN NEOPLASM OF FALLOPIAN TUBE AND UTERINE LIGAMENTS - 221.9 BENIGN NEOPLASM -

Updates to ICD-10-CM Take Effect October 1, 2016
Changes in Family Planning Coding: Updates to ICD-10-CM Take Effect October 1, 2016 Family planning providers will soon have access to new diagnosis codes, including those that better reflect the full range of contraceptive methods currently available on the market. The Centers for Medicare & Medicaid Services (CMS) and the Centers for Disease Control and Prevention's (CDC) National Center for Health Statistics (NCHS) reviewed the International Classification of Diseases, 10th Edition (ICD-10) for use in the US and published changes that will take effect on October 1, 2016. This set of updates are collectively known as the 2017 Addenda and are to be used for patient encounters occurring from October 1, 2016 through September 30, 2017. The 2017 Addenda includes two types of updates: 1) new codes added to ICD-10-CM and 2) edits to descriptive language of current ICD-10-CM codes that will clarify use of codes. Both updates are important for providers to understand. A new code may better represent health care services that are currently provided and should therefore be incorporated into use. A new description for a code currently in use may provide better insight into the appropriate use of codes, or may correct an error in a description that created confusion. The following pages include a list of changes to ICD-10-CM that are pertinent to family planning providers. The document is divided into three sections: 1) new ICD-10-CM codes that may be needed frequently in family planning settings; 2) new ICD-10-CM codes related to reproductive health but used infrequently in family planning settings; and 3) edits to ICD-10- CM tabular list descriptions among codes of interest to family planning providers. -

CTRI Trial Data
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Thu, 30 Sep 2021 12:17:01 GMT) CTRI Number CTRI/2020/07/026410 [Registered on: 07/07/2020] - Trial Registered Prospectively Last Modified On 06/07/2020 Post Graduate Thesis Yes Type of Trial Interventional Type of Study Drug Medical Device Ayurveda Study Design Randomized, Parallel Group Trial Public Title of Study Clinical Study To Assess The Efficacy of Ayurvedic Formulations In PCOS Scientific Title of A Randomized Control Open Label Clinical Trial On The Efficacy Of Shatapushpa Churna And Study Madhutailika Basti In Nashtartava w.s.r. To Polycystic Ovarian Syndrome Secondary IDs if Any Secondary ID Identifier NIL NIL Details of Principal Details of Principal Investigator Investigator or overall Name Dr Shalvi Sharma Trial Coordinator (multi-center study) Designation M.S. Scholar Affiliation National Institute of Ayurveda, Jaipur Address Department of Prasuti Tantra evam Stri Roga National Institute of Ayurveda Madhav Villas Palace Jorawar Singh Gate Amer road Jaipur RAJASTHAN 302020 India Phone 8988071274 Fax Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr K Bharathi Query) Designation Professor and H.O.D. Affiliation National Institute of Ayurveda, Jaipur Address Department of Prasuti Tantra evam Stri Roga National Institute of Ayurveda Madhav Villas Palace Jorawar Singh Gate Amer road Jaipur RAJASTHAN 302020 India Phone 9982678508 Fax Email [email protected] Details Contact -
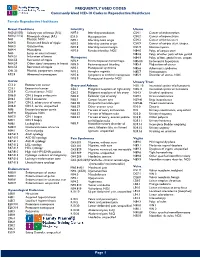
Commonly Used ICD-10 Codes in Reproductive Healthcare
FREQUENTLY USED CODES Commonly Used ICD-10 Codes in Reproductive Healthcare Female Reproductive Healthcare Breast Conditions Infertility Uterus N60.(01/02) Solitary cyst of breast (R/L) N97.0 Infertility-anovulation C54.1 Cancer of endometrium N60.(11/12) Fibrocystic change (R/L) E23.0 Hypopituarism C54.2 Cancer of myometrium N61 Mastitis, NOS N97.1 Infertility-tubal origin C54.3 Cancer of fundus uteri N64.0 Fissure and fistula of nipple N97.2 Infertility-uterine origin C54.9 Cancer of corpus uteri, unspec. N64.3 Galactorrhea N97.8 Infertility-cervical origin D25.9 Uterine myoma N64.4 Mastodynia N97.9 Female infertility, NOS N84.0 Polyp of corpus uteri N63 Lump or mass in breast N84.8 Polyp of other parts of fem genital N64.51 Induration of breast Menopause N84.9 Polyptract of fem. genital tract, unspec. N64.53 Retraction of nipple N92.4 Perimenopausal menorrhagia N85.00 Endometrial hyperplasia N64.59 Other signs/ symptoms in breast N95.0 Postmenopausal bleeding N85.4 Malposition of uterus N64.53 Retraction of nipple N95.1 Menopausal syndrome N85.6 Asherman’s syndrome O91.23 Mastitis, postpartum, unspec. N95.2 Atrophic vaginitis N85.7 Hematometra R92.8 Abnormal mammogram N95.8 Symptoms w artificial menopause N85.9 Disorder of uterus, NOS N95.9 Menopausal disorder NOS Cervix Urinary Tract C53.0 Endocervical cancer Ovary and Adnexa N30. 10 Interstitial cystitis w/o hematuria C53.1 Exocervical cancer C56.1 Malignant neoplasm of right ovary N30.11 Interstitial cystitis w/ hematuria C53.9 Cervical cancer, NOS C56.2 Malignant neoplasm of left -

Errata to the 10/1/2016 Prioritized List
Errata to the 10/1/2016 Prioritized List 1) On 9/13/2017 Guideline Note 144 was corrected to reference line 385 rather than line 516 in the last paragraph. The corrected text reads as follows: a. Long term proton pump inhibitor therapy is included on Line 385 for Barrett’s esophagus (ICD-10-CM K22.70). 2) On 6/29/2017, the following corrections were made: a. Website addresses were corrected for the new Oregon Health Authority Web site in all guideline notes referencing a Coverage Guidance. In addition, a web address in the Multisector Interventions section on Tobacco Use was updated. b. M67.0 (Short Achilles tendon (acquired)) was moved to line 297 NEUROLOGICAL DYSFUNCTION IN POSTURE AND MOVEMENT CAUSED BY CHRONIC CONDITIONS from line 382 DYSFUNCTION RESULTING IN LOSS OF ABILITY TO MAXIMIZE LEVEL OF INDEPENDENCE IN SELF- DIRECTED CARE CAUSED BY CHRONIC CONDITIONS THAT CAUSE NEUROLOGICAL DYSFUNCTION c. G56.23 (Lesion of ulnar nerve, bilateral upper limbs) was moved from lines 512 PERIPHERAL NERVE DISORDERS and 539 PERIPHERAL NERVE DISORDERS to line 421 PERIPHERAL NERVE ENTRAPMENT; PALMAR FASCIAL FIBROMATOSIS. d. Psoriasis corrections: i. Add psoriasis, parapsoriasis and similar ICD-10 codes to line 544 MILD PSORIASIS; DERMATOPHYTOSIS: SCALP, HAND, BODY, DEEP-SEATED 1. L40.0 Psoriasis vulgaris 2. L40.1 Generalized pustular psoriasis 3. L40.2 Acrodermatitis continua 4. L40.3 Pustulosis palmaris et plantaris 5. L40.4 Guttate psoriasis 6. L40.8 Other psoriasis 7. L40.9 Psoriasis, unspecified 8. L41.0 Pityriasis lichenoides et varioliformis acuta ii. Add psoriatic arthropathy ICD-10 codes to line 50 RHEUMATOID ARTHRITIS AND OTHER INFLAMMATORY POLYARTHROPATHIES) and remove from line SEVERE INFLAMMATORY SKIN DISEASE 1. -

GYN Coding Changes to Note for Your Maximized Reimbursement Revised ICD-10 Gynecologic Diagnostic Codes Go Into Effect October 1
Reimbursement ADVISER GYN coding changes to note for your maximized reimbursement Revised ICD-10 gynecologic diagnostic codes go into effect October 1. Here is a look at the added, expanded, and revised codes you will need for your practice. Melanie Witt, RN, MA n the August 2016 issue of OBG Manage- requested new codes to align with a 2009 ment, I wrote about the International joint report on the terminology for female IClassification of Diseases, Tenth Revi- pelvic floor dysfunction.1 These codes, along sion, Clinical Modification (ICD-10) coding with others, are listed in TABLE 1, page e2. changes that will occur for obstetric services, and now it is time to tackle gynecologic ser- Urinary procedure complication codes vices. The changes cover the gamut of issues Not every urogynecologist will have an issue that could not be addressed with the initial after surgery for incontinence, but if they IN THIS implementation of ICD-10, including codes do, there are tons of new and revised codes ARTICLE for contraception, prepubertal issues, post- to address every possible complication the operative complications, and urinary symp- patient may have (TABLE 2, page e3). Each of Urogynecology toms, to name a few. these codes is reported based on whether the dx codes complication is being actively treated (initial page e2 encounter: final character is A), is being fol- Urogynecology lowed up after treatment (subsequent encoun- diagnostic codes ter: final character is D), or is caused by another Gynecology Urogynecologists will find a large num- condition (sequela: final character is S). dx codes ber of changes to codes they can select on page e5 October 1, 2016. -

Diseases of the Female Reproductive System. Part I. Methodical
MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE State Higher Educational Institution “UZHGOROD NATIONAL UNIVERSITY” MEDICAL FACULTY DEPARTMENT OF OBSTETRICS AND GYNECOLOGY Authors: As.Prof. Lemish N.Y. Ph.D, prof. Korchynska O.O. Topic: Diseases of the female reproductive system. Part I. Methodical development for practical lessons of gynecology for students of the 5th course of medical faculty Uzhhgorod - 2015 Methodical development was prepared by: N.Y. Lemish – assistant professor of the department of obstetrics and gynecology of medical faculty, Uzhgorod national university O.O. Korchynska – doctor of medical sciences, professor of department of obstetrics and gynecology, medical faculty, Uzhgorod national university Edited by: chief of the department of obstetrics and gynecology state higher educational institution “Uzhgorod national university”, doctor of medical sciences, prof. Malyar V.A. Reviewers: Y.Y. Bobik – doctor of medical sciences, professor, the head of department of maternal and neonatal care, faculty of postgraduate study, State higher educational instuitution “Uzhgorod national university” S.O.Herzanych - doctor of medical sciences, professor of department of obstetrics and gynecology, medical faculty, State higher educational institution “Uzhgorod national university” Approved by the Academic Council of the medical faculty, protocol № 7 from « 19 » march 2015 year. 2 ABBREVIATIONS MC – Menstrual cycle FSH - Follicle Stimulating Hormone AMN - Anti –Mullerian Hormone LH – Luteinizing hormone DUB – dysfunctional uterine -

ICD-10: Clinical Concepts for OB/GYN
Clinical Concepts ICD-10 for OB/GYN ICD-10 Clinical Concepts Series Common Codes Clinical Documentation Tips Clinical Scenarios ICD-10 Clinical Concepts for OB/GYN is a feature of Road to 10, a CMS online tool built with physician input. With Road to 10, you can: l Build an ICD-10 action plan customized for l Access quick references from CMS and your practice medical and trade associations l Use interactive case studies to see how l View in-depth webcasts for and by medical your coding selections compare with your professionals peers’ coding To get on the Road to 10 and find out more about ICD-10, visit: cms.gov/ICD10 roadto10.org ICD-10 Compliance Date: October 1, 2015 Official CMS Industry Resources for the ICD-10 Transition www.cms.gov/ICD10 1 Table Of Contents Common Codes • Abnormal Female Genital • Selected Menopausal and Cytology (Excluding other Perimenopausal Neoplasia and Malignancy Disorders Codes) • Noninflammatory Disorders • Excessive, Frequent and of Ovary, Fallopian Tubes Irregular Menstruation and Broad Ligament • General Medical and • Supervision of Normal Gynecological Examinations Pregnancy • Hypertension • Urinary Tract Infection, • Inflammation of Vagina and Cystitis Vulva • Lump in Breast and Other Disorders of Breast Primer for Clinical Documentation • Trimester • Intent of Encounter • Vomiting • Complications of Pregnancy • Abortion • Alcohol Use, Substance Abuse and Tobacco • Childbirth and Puerperium Dependence Distinct from Trimester Clinical Scenarios • Scenario 1: Abdominal Pain & Ovarian Cyst • Scenario -

General Ultrasound
SCHAFFER, SCHONHOLZ & DROSSMAN, LLP 488 Madison Avenue Suite 1220 New York, NY 10022 • Tel 212.755.7656 Fax 212.688.9474 PATIENT REFERRAL INFORMATION GENERAL ULTRASOUND Patient’s Name: Patient’s DOB: Patient’s Medicare#: Referring Physician’s Name & Address: Referring Physician’s Signature: Date: X CODE ABDOMINAL ULTRASOUND X CODE ABDOMINAL ULTRASOUND (cont’d) X CODE RENAL SONOGRAPHY 76700 Abdominal Ultrasound R19.01 Right upper quadrant abdominal swell, mass & lump 76770 Renal Ultrasound R10.819 Abdominal tenderness, unspec site R10.811 Right upper quadrant abdominal tenderness N30.01 Acute cystitis w/hematuria R94.5 Abnormal results of liver function studies R10.11 Right upper quadrant pain R31.1 Benign essential microscopic hematuria R10.0 Acute abdomen R10.821 Right upper quadrant rebound abdominal tenderness N20.0 Calculus of Kidney K80.18 Calculus of gallbladder w/other cholecystitis w/o obstr R10.9 Unspec abdominal pain N28.1 Cyst of kidney, acquired R10.83 Colic R10.10 Upper abdominal pain, unspec N30.91 Cystitis, unspec w/hematuria K57.93 Diverticulitis intest, part unsp w/o perf/abscess w/bld R31.0 Gross hematuria K57.92 Diverticulitis intest, part unsp w/o perf/abscess w/o bld R31.9 Hematuria Unspec K57.80 Diverticulitis intest, part unsp w/perf/abscess w/o bld X CODE PELVIC ULTRASOUND N99.521 Infection of other external stoma of urinary tract K57.32 Diverticulitis lrg intest w/o perf/abscess w/o bleed 76830 Pelvic Transvaginal Ultrasound N99.531 Infection of other stoma of urinary tract K57.81 Diverticulitis of intest, part -

Icd-10Causeofdeath.Pdf
A00 Cholera A00.0 Cholera due to Vibrio cholerae 01, biovar cholerae A00.1 Cholera due to Vibrio cholerae 01, biovar el tor A00.9 Cholera, unspecified A01 Typhoid and paratyphoid fevers A01.0 Typhoid fever A01.1 Paratyphoid fever A A01.2 Paratyphoid fever B A01.3 Paratyphoid fever C A01.4 Paratyphoid fever, unspecified A02 Other salmonella infections A02.0 Salmonella gastroenteritis A02.1 Salmonella septicemia A02.2 Localized salmonella infections A02.8 Other specified salmonella infections A02.9 Salmonella infection, unspecified A03 Shigellosis A03.0 Shigellosis due to Shigella dysenteriae A03.1 Shigellosis due to Shigella flexneri A03.2 Shigellosis due to Shigella boydii A03.3 Shigellosis due to Shigella sonnei A03.8 Other shigellosis A03.9 Shigellosis, unspecified A04 Other bacterial intestinal infections A04.0 Enteropathogenic Escherichia coli infection A04.1 Enterotoxigenic Escherichia coli infection A04.2 Enteroinvasive Escherichia coli infection A04.3 Enterohemorrhagic Escherichia coli infection A04.4 Other intestinal Escherichia coli infections A04.5 Campylobacter enteritis A04.6 Enteritis due to Yersinia enterocolitica A04.7 Enterocolitis due to Clostridium difficile A04.8 Other specified bacterial intestinal infections A04.9 Bacterial intestinal infection, unspecified A05 Other bacterial food-borne intoxications A05.0 Food-borne staphylococcal intoxication A05.1 Botulism A05.2 Food-borne Clostridium perfringens [Clostridium welchii] intoxication A05.3 Food-borne Vibrio parahemolyticus intoxication A05.4 Food-borne Bacillus cereus -

Definitions of Medicare Code Edits
Preliminary 08/25/09 Definitions of Medicare Code Edits PBL–011 October 2009 08/25/09 Preliminary Document number PBL–011 October 2009 ii Definitions of Medicare Code Edits October 2009 Preliminary 08/25/09 About this document THE MEDICARE CODE EDITOR (MCE) detects and reports errors in the coding claims data. This manual contains a description of each coding edit with corresponding ICD-9-CM code lists. The coding edit information in this manual is effective from 10/01/2009 to 09/30/2010. October 2009 iii 08/25/09 Preliminary iv Definitions of Medicare Code Edits October 2009 Preliminary 08/25/09 Contents Preface About this document iii Chapter 1 Edit code lists 1.3 1. Invalid diagnosis or procedure code 1.4 2. E-code as principal diagnosis 1.4 3. Duplicate of PDX 1.4 4. Age conflict 1.4 5. Sex conflict 1.19 6. Manifestation code as principal diagnosis 1.38 7. Non-specific principal diagnosis 1.41 8. Questionable admission 1.41 9. Unacceptable principal diagnosis 1.42 10. Non-specific O.R. procedure 1.50 11. Non-covered procedure 1.50 12. Open biopsy check 1.53 13. Bilateral procedure 1.54 14. Invalid age 1.55 15. Invalid sex 1.55 16. Invalid discharge status 1.55 17. Limited coverage 1.56 18. Wrong procedure performed 1.57 Chapter 2 Code list changes 2.3 Changes to existing edits 2.3 New edit 2.13 Index I.1 October 2009 Contents v 08/25/09 Preliminary vi Definitions of Medicare Code Edits October 2009 Chapter 1 1Edit code lists Contents Edit code lists 1.3 1. -

Laparoscopic Excision of Tubes and Ovaries Hs-263
LAPAROSCOPIC EXCISION OF TUBES AND OVARIES HS-263 Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. ‘Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois, Inc. WellCare Health Plans of New Jersey, Inc. WellCare Health Insurance of Arizona, Inc. WellCare of Florida, Inc. WellCare of Connecticut, Inc. WellCare of Georgia, Inc. WellCare of Kentucky, Inc. WellCare of Louisiana, Inc. WellCare of New York, Inc. Laparoscopic Excision WellCare of South Carolina, Inc. of Tubes and Ovaries WellCare of Texas, Inc. Policy Number: HS-263 WellCare Prescription Insurance, Inc. Original Effective Date: 8/7/2014 Windsor Health Plan Windsor Rx Medicare Prescription Drug Plan Revised Date(s): 7/11/2015 APPLICATION STATEMENT The application of the Clinical Coverage Guideline is subject to the benefit determinations set forth by the Centers for Medicare and Medicaid Services (CMS) National and Local Coverage Determinations and state-specific Medicaid mandates, if any. Clinical Coverage Guideline page 1 Original Effective Date: 8/7/2014 - Revised: 7/11/2015 LAPAROSCOPIC EXCISION OF TUBES AND OVARIES HS-263 DISCLAIMER The Clinical Coverage Guideline is intended to supplement certain standard WellCare benefit plans. The terms of a member’s particular Benefit Plan, Evidence of Coverage, Certificate of Coverage, etc., may differ significantly from this Coverage Position. For example, a member’s benefit plan may contain specific exclusions related to the topic addressed in this Clinical Coverage Guideline. When a conflict exists between the two documents, the Member’s Benefit Plan always supersedes the information contained in the Clinical Coverage Guideline.