SPECIFIC ANTIDOTES Administered After Toxicant Absobtion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surfen, a Small Molecule Antagonist of Heparan Sulfate
Surfen, a small molecule antagonist of heparan sulfate Manuela Schuksz*†, Mark M. Fuster‡, Jillian R. Brown§, Brett E. Crawford§, David P. Ditto¶, Roger Lawrence*, Charles A. Glass§, Lianchun Wang*, Yitzhak Torʈ, and Jeffrey D. Esko*,** *Department of Cellular and Molecular Medicine, Glycobiology Research and Training Center, †Biomedical Sciences Graduate Program, ‡Department of Medicine, Division of Pulmonary and Critical Care Medicine and Veteran’s Administration San Diego Medical Center, ¶Moores Cancer Center, and ʈDepartment of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA 92093; and §Zacharon Pharmaceuticals, Inc, 505 Coast Blvd, South, La Jolla, CA 92037 Communicated by Carolyn R. Bertozzi, University of California, Berkeley, CA, June 18, 2008 (received for review May 26, 2007) In a search for small molecule antagonists of heparan sulfate, Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was first we examined the activity of bis-2-methyl-4-amino-quinolyl-6- described in 1938 as an excipient for the production of depot carbamide, also known as surfen. Fluorescence-based titrations insulin (16). Subsequent studies have shown that surfen can indicated that surfen bound to glycosaminoglycans, and the extent block C5a receptor binding (17) and lethal factor (LF) produced of binding increased according to charge density in the order by anthrax (18). It was also reported to have modest heparin- heparin > dermatan sulfate > heparan sulfate > chondroitin neutralizing effects in an oral feeding experiments in rats (19), sulfate. All charged groups in heparin (N-sulfates, O-sulfates, and but to our knowledge, no further studies involving heparin have carboxyl groups) contributed to binding, consistent with the idea been conducted, and its effects on HS are completely unknown. -

Review of Succimer for Treatment of Lead Poisoning
Review of Succimer for treatment of lead poisoning Glyn N Volans MD, BSc, FRCP. Department of Clinical Pharmacology, School of Medicine at Guy's, King's College & St Thomas' Hospitals, St Thomas' Hospital, London, UK Lakshman Karalliedde MB BS, DA, FRCA Consultant Medical Toxicologist, CHaPD (London), Health Protection Agency UK, Visiting Senior Lecturer, Division of Public Health Sciences, King's College Medical School, King's College , London Senior Research Collaborator, South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, Peradeniya, Sri Lanka. Heather M Wiseman BSc MSc Medical Toxicology Information Services, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, UK. Contact details: Heather Wiseman Medical Toxicology Information Services Guy’s & St Thomas’ NHS Foundation Trust Mary Sheridan House Guy’s Hospital Great Maze Pond London SE1 9RT Tel 020 7188 7188 extn 51699 or 020 7188 0600 (admin office) Date 10th March 2010 succimer V 29 Nov 10.doc last saved: 29-Nov-10 11:30 Page 1 of 50 CONTENTS 1 Summary 2. Name of the focal point in WHO submitting or supporting the application 3. Name of the organization(s) consulted and/or supporting the application 4. International Nonproprietary Name (INN, generic name) of the medicine 5. Formulation proposed for inclusion 6. International availability 7. Whether listing is requested as an individual medicine or as an example of a therapeutic group 8. Public health relevance 8.1 Epidemiological information on burden of disease due to lead poisoning 8.2 Assessment of current use 8.2.1 Treatment of children with lead poisoning 8.2.2 Other indications 9. -

Guideline on Antidote Availability for Emergency Departments January 2017
Royal College of Emergency Medicine and National Poisons Information Service Guideline on Antidote Availability for Emergency Departments January 2017 TOXBASE and/or the BNF should be consulted for further advice on doses and indications for antidote administration and, if necessary, the National Poisons Information Service (NPIS) should be telephoned for more patient-specific advice. Contact details for NPIS are available on TOXBASE. Additional drugs that are used in the poisoned patient that are widely available in ED are not listed in the table – in particular it is important to ensure that insulin, benzodiazepines (diazepam and/or lorazepam), glyceryl trinitrate or isosorbide dinitrate and magnesium are immediately available in the ED. The following drugs should be immediately available in the ED or any area where poisoned patients are initially treated. These drugs should be held in a designated storage facility* The stock held should be sufficient to initiate treatment (stocking guidance is in Appendix 1). Drug Indication Acetylcysteine Paracetamol Activated charcoal Many oral poisons Atropine Organophosphorus or carbamate insecticides Bradycardia Calcium chloride Calcium channel blockers Systemic effects of hydrofluoric acid Calcium gluconate Local infiltration for hydrofluoric acid Calcium gluconate gel Hydrofluoric acid Cyanide antidotes Cyanide Dicobalt edetate The choice of antidote depends on the severity of poisoning, certainty of diagnosis and cause Hydroxocobalamin (Cyanokit®) of poisoning/source of cyanide. Sodium nitrite - Dicobalt edetate is the antidote of choice in severe cases when there is a high clinical Sodium thiosulphate suspicion of cyanide poisoning e.g. after cyanide salt exposure. - Hydroxocobalamin (Cyanokit®) should be considered in smoke inhalation victims who have a severe lactic acidosis, are comatose, in cardiac arrest or have significant cardiovascular compromise - Sodium nitrite may be used if dicobalt edetate is not available. -

Uses and Administration Adverse Effects and Precautions Pharmacokinetics Uses and Administration
1549 The adverse effects of dicobalt edetate are more severe in year-old child: case report and review of literature. Z Kardiol 2005; 94: References. 817-2 3. the absence of cyanide. Therefore, dicobalt edetate should I. Schaumann W, et a!. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur 1 not be given unless cyanide poisoning is confirmed and 30: Administration in renal impairment. In renal impairment, Clin Pharmacol 1986; 527-33. poisoning is severe such as when consciousness is impaired. 2. Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab elimination of antibody-bound digoxin or digitoxin is therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; Oedema. A patient with cyanide toxicity developed delayed1·3 and antibody fragments can be detected in the 28: 483-93. plasma for 2 to 3 weeks after treatment.1 The rebound in 3. Renard C, et al. Pharmacokinetics of dig,'><i<1-sp,eei1ie Fab: effects of severe facial and pulmonary oedema after treatment with decreased renal function and age. 1997; 44: 135-8. dicobalt edetate.1 It has been suggested that when dicobalt free-digoxin concentrations that has been reported after edetate is used, facilities for intubation and resuscitation treatment with digoxin-specific antibody fragments (see P (Jrations should be immediately available. Poisoning, below), occurred much later in patients with r.�p renal impairment than in those with normal renal func Proprietary Preparations (details are given in Volume B) 1. Dodds C, McKnight C. Cyanide toxicity after immersion and the hazards tion. -

RCEM NPIS Antidote Guideline Appx 1
Royal College of Emergency Medicine and National Poisons Information Service Guideline on Antidote Availability for Emergency Departments (January 2017) Appendix 1. Stock levels & storage recommendations Doses and Clinical Advice on the Administration of Antidotes TOXBASE and/or the BNF should be consulted for further advice on doses and indications for antidote administration. If necessary, the National Poisons Information Service (NPIS) should be telephoned for more patient-specific advice. Contact details for NPIS are available on TOXBASE. Stock Levels The recommended minimum stocking levels (rounded up to full “pack-sizes” where necessary) are based on the amount of antidote required to initiate treatment for an adult patient in the ED and to continue treatment for the first 24 hours. Higher stock levels may be required and individual departments should determine the amount of each antidote they stock based on the epidemiology of poisoning presentations to their department. Additional drugs that are used in the poisoned patient that are widely available in ED are not listed in the table – in particular it is important to ensure that insulin, benzodiazepines (diazepam and/or lorazepam), glyceryl trinitrate or isosorbide mononitrate and magnesium are immediately available in the ED. The following drugs should be immediately available in the ED or any area where poisoned patients are initially treated These drugs should be held in a designated storage facility Recommended stock Drug Indication Presentation Special storage conditions -

WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T (51) International Patent Classification: (74) Agents: CHOTIA, Meenakshi et al; K&S Partners | Intel A 25/12 (2006.01) A61K 8/11 (2006.01) lectual Property Attorneys, 4121/B, 6th Cross, 19A Main, A 25/34 (2006.01) A61K 8/49 (2006.01) HAL II Stage (Extension), Bangalore 560038 (IN). A01N 37/06 (2006.01) A61Q 5/00 (2006.01) (81) Designated States (unless otherwise indicated, for every A O 43/12 (2006.01) A61K 31/44 (2006.01) kind of national protection available): AE, AG, AL, AM, AO 43/40 (2006.01) A61Q 19/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A01N 57/12 (2006.01) A61K 9/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, AOm 59/16 (2006.01) A61K 31/496 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/IB20 14/06 1925 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 3 June 2014 (03.06.2014) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Thiosulfate De Sodium Sterop
Thiosulfate de Sodium Sterop 1. DENOMINATION DU MEDICAMENT THIOSULFATE DE SODIUM STEROP 1g/5ml solution injectable 2. COMPOSITION QUALITATIVE ET QUANTITATIVE Substance active : Une ampoule de 5ml contient 1g de thiosulfate de sodium dans de l’eau pour préparations injectables. 1 ml de solution contient 200mg de thiosulfate de sodium. Pour la liste complète des excipients, voir rubrique 6.1. 3. FORME PHARMACEUTIQUE Solution injectable. 4. DONNEES CLINIQUES 4.1 Indications thérapeutiques - Antidote dans le traitement des intoxications par les cyanures ainsi que par le nitroprussiate de sodium. - Prévention des effets néphrotoxiques du cisplatine. Thiosulfate de Sodium Sterop © Pharma.be Pagina 1 van 6 4.2 Posologie et mode d’administration Mode d'administration Pour voie intraveineuse lente. Posologie Intoxications par les cyanures: Adultes: La posologie habituelle est de 12,5 g de thiosulfate de sodium, administré par voie I.V. sur une période de 5 à 10 minutes. Enfants: La posologie habituelle est de 412,5 mg de thiosulfate de sodium par kg, administré par voie I.V. à la vitesse de 0,625 à 1,25 g/minute sur une période de 5 à 10 minutes. Si les symptômes persistent ou réapparaissent dans la demi-heure ou dans l’heure ayant suivi la première injection, une seconde injection de thiosulfate de sodium est nécessaire avec comme posologie la moitié de celle de la première injection. En prévention des effets néphrotoxiques du cisplatine: Chez l'adulte, la posologie initiale est de 9 g de thiosulfate de sodium par m2 de surface corporelle, administré par voie I.V. Elle est suivie par l'injection de 1,2 g de thiosulfate de sodium par m2 de surface corporelle par heure, en perfusion I.V. -
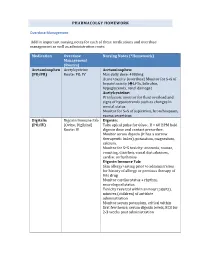
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Fomepizole for Acetaminophen Toxicity
Clinical Pharmacology & Toxicology Pearl of the Week Fomepizole for Acetaminophen Toxicity Mechanisms There are two ways that fomepizole works to help prevent hepatotoxicity in APAP overdose. The primary mechanism involves inhibition of CYP450 2E1. o When a toxic amount of APAP is ingested, the pathways that convert it into nontoxic metabolites become overwhelmed. o Thus, more APAP gets converted into NAPQI. o Fomepizole inhibits the 2E1 pathway, thus preventing conversion of APAP into NAPQI. The second mechanism of action is the JNK (“junk”) enzyme. o NAPQI-induced mitochondrial dysfunction leads to formation of reactive oxidant species. This oxidant stress leads to activation of c-jun N-terminal kinase, or JNK, enzyme. o This enzyme translocates to the mitochondria, amplifying oxidant stress, and ultimately resulting in the cessation of ATP production. o Additionally, the JNK enzyme can lead to the rupture of the outer mitochondrial membrane, causing release of intermembrane proteins, which can potentially lead to DNA fragmentation o Interestingly, it is thought that both metabolic acidosis, as well as elevated lactate levels, may be the result of alterations in mitochondrial respiratory function o Fomepizole works by preventing the APAP-induced activation of this enzyme. Role in Acetaminophen poisoning management In cases where fomepizole and NAC were administered together, evidence has shown that the combination has contributed to a decrease in both hepatotoxicity and mortality. Most of the evidence has come from case reports and animal models. Currently, fomepizole is recommended for massive APAP ingestions (as in, ingestions over 500- 600 mg/kg or in cases where 4-hour equivalent acetaminophen concentrations over 5000 umol/L). -

Chelating Drug Therapy: an Update
Open Access Austin Journal of Genetics and Genomic Research Review Article Chelating Drug Therapy: An Update Vijay Kumar1, Ashok Kumar2*, Sandeep Kumar Singh1, Manoj Kumar3, Surendra Kumar2, Dinesh Abstract 4 5 Kumar and Ragni Singh Purpose: To study the clinical effects of metal toxicity and current 1Department of Neurology, SGPGIMS, India recommendations for management, including chelation therapy, are reviewed. 2Department of Medical Genetics, SGPGIMS, India 3Department of Microbiology, SGPGIMS, India Summary: Metals are essential to many biological processes, but excess 4Department of Chemistry, Dr. R.M.L. Avadh University, of it becomes hazardous to life. These are necessary for cell growth, electron India transport chain, several enzymatic activities and response of immune systems. 5Bheem Rao Ambedkar Bihar University, India They also serve as a cofactor for several enzymes. Chelation therapy is used for clinical management of the excess of metal. However, each metal requires *Corresponding author: Ashok Kumar, Department a specific chelation agent. A chelate is a compound form between metal and a of Medical Genetics, Sanjay Gandhi Post Graduate compound that contains two or more potential ligands. A promising Fe chelator Institute of Medical Sciences, Lucknow, India is Desferrioxamine (Desferal). Penicillamine and Trientine are uses for copper Received: March 12, 2015; Accepted: April 24, 2015; chelation. Meso-2,3-Dimercaptosuccinic Acid (DMSA) and 2,3-Dimercapto- Published: April 27, 2015 Propanesulphonate (DMPS) can be used as effective chelator of mercury. Dimercaprol, edetate calcium disodium, and succimer are the three agents primarily used for chelation of lead. Conclusion: Metal toxicity remains a significant public health concern. Elimination of elevated metal ions can be achieved by proper chelation agents. -

Changes in Tissue Gadolinium Biodistribution Measured in an Animal Model Exposed to Four Chelating Agents
Japanese Journal of Radiology (2019) 37:458–465 https://doi.org/10.1007/s11604-019-00835-1 ORIGINAL ARTICLE Changes in tissue gadolinium biodistribution measured in an animal model exposed to four chelating agents Türker Acar1,6 · Egemen Kaya2 · Mustafa Deniz Yoruk3 · Neslihan Duzenli4 · Recep Selim Senturk4 · Cenk Can4 · Lokman Ozturk3 · Canberk Tomruk5 · Yigit Uyanikgil5 · Frank J. Rybicki6 Received: 17 December 2018 / Accepted: 22 March 2019 / Published online: 30 March 2019 © Japan Radiological Society 2019 Abstract Purpose This study investigated the potential to reduce gadolinium levels in rodents after repetitive IV Gadodiamide admin- istration using several chelating agents. Materials and methods The following six groups of rats were studied. Group 1: Control; Group 2: Gadodiamide only; Group 3: Meso-2,3-Dimercaptosuccinic acid (DMSA) + Gadodiamide; Group 4: N-Acetyl-L-cysteine (NAC) + Gadodiamide; Group 5: Coriandrum sativum extract + Gadodiamide; and Group 6: Deferoxamine + Gadodiamide. Brain, kidney, and blood samples were evaluated via inductively coupled plasma mass spectrometry. The brain was also evaluated histologically. Results Kidney gadolinium levels in Groups 4 and 5 were approximately double that of Group 2 (p = 0.033 for each). There was almost no calcifcation in rat hippocampus for Group 4 rodents when compared with Groups 2, 3, 5 and 6. Conclusion Our preliminary study shows that excretion to the kidney has a higher propensity in NAC and Coriandrum sati- vum groups. It may be possible to change the distribution of gadolinium by administrating several agents. NAC may lower Gadodiamide-induced mineralization in rat hippocampus. Keywords Gadolinium deposition · Dimercaptosuccinic acid · N-Acetyl-L-cysteine · Coriandrum sativum · Deferoxamine Introduction However, the safety profle of gadolinium-based agents [1] was questioned after the discovery of nephrogenic sys- Gadolinium-based contrast agents (GBCA) have been safely temic fbrosis [2], a scleroderma-like disease characterized used in diagnostic radiology since the 1980s. -

Adult BLS Standing Orders • Ensure EMS Provider Safety, Consider HAZMAT Activation
Imperial County Public Health Department Emergency Medical Services Agency Policy/Procedure/Protocol Manual Treatment Protocols Date: 07/01/2021 Poisoning/Intoxication/Envenomation - Adult Policy #9160A Adult BLS Standing Orders • Ensure EMS provider safety, consider HAZMAT activation. Recognize, Notify, Isolate • Universal Patient Protocol • Do not approach patient or location if scene safety is in question • Obtain accurate history of incident: o Name of product or substance o Quantity ingested, and/or duration of exposure o Time elapsed since exposure o If safe and accessible, bring medications or bottles to hospital • Move victim(s) to safe environment • Externally decontaminate - PRN • Continuously monitor ECG, blood pressure, pulse oximetry, and capnography (if ALS present) PRN • Give oxygen and provide airway support per Airway Policy • Contact Poison Control Center as needed 1 (800) 222-1222 Suspected Opioid Overdose with Respirations <12 RPM • If possible, avoid the use of a supraglottic device prior to the administration of naloxone • Administer naloxone 0.1 mg/kg, max of 2 mg IN. May repeat up to three (3) times, q5min • May assist family/friends on-scene with administration of patient’s own naloxone • NOTE - Use with caution in opioid dependent pain management patients • Assess vitals, with specific attention to respiratory rate and respiratory drive • Note pupil exam • Note drug paraphernalia or medication bottles near patient Suspected Stimulant Overdose with Sudden Hypoventilation, Oxygen Desaturation, or Apnea • High flow