Concepts and Approaches for Mars Exploration 6194.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
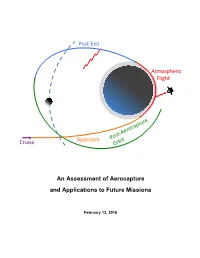
An Assessment of Aerocapture and Applications to Future Missions
Post-Exit Atmospheric Flight Cruise Approach An Assessment of Aerocapture and Applications to Future Missions February 13, 2016 National Aeronautics and Space Administration An Assessment of Aerocapture Jet Propulsion Laboratory California Institute of Technology Pasadena, California and Applications to Future Missions Jet Propulsion Laboratory, California Institute of Technology for Planetary Science Division Science Mission Directorate NASA Work Performed under the Planetary Science Program Support Task ©2016. All rights reserved. D-97058 February 13, 2016 Authors Thomas R. Spilker, Independent Consultant Mark Hofstadter Chester S. Borden, JPL/Caltech Jessie M. Kawata Mark Adler, JPL/Caltech Damon Landau Michelle M. Munk, LaRC Daniel T. Lyons Richard W. Powell, LaRC Kim R. Reh Robert D. Braun, GIT Randii R. Wessen Patricia M. Beauchamp, JPL/Caltech NASA Ames Research Center James A. Cutts, JPL/Caltech Parul Agrawal Paul F. Wercinski, ARC Helen H. Hwang and the A-Team Paul F. Wercinski NASA Langley Research Center F. McNeil Cheatwood A-Team Study Participants Jeffrey A. Herath Jet Propulsion Laboratory, Caltech Michelle M. Munk Mark Adler Richard W. Powell Nitin Arora Johnson Space Center Patricia M. Beauchamp Ronald R. Sostaric Chester S. Borden Independent Consultant James A. Cutts Thomas R. Spilker Gregory L. Davis Georgia Institute of Technology John O. Elliott Prof. Robert D. Braun – External Reviewer Jefferey L. Hall Engineering and Science Directorate JPL D-97058 Foreword Aerocapture has been proposed for several missions over the last couple of decades, and the technologies have matured over time. This study was initiated because the NASA Planetary Science Division (PSD) had not revisited Aerocapture technologies for about a decade and with the upcoming study to send a mission to Uranus/Neptune initiated by the PSD we needed to determine the status of the technologies and assess their readiness for such a mission. -

Up, Up, and Away by James J
www.astrosociety.org/uitc No. 34 - Spring 1996 © 1996, Astronomical Society of the Pacific, 390 Ashton Avenue, San Francisco, CA 94112. Up, Up, and Away by James J. Secosky, Bloomfield Central School and George Musser, Astronomical Society of the Pacific Want to take a tour of space? Then just flip around the channels on cable TV. Weather Channel forecasts, CNN newscasts, ESPN sportscasts: They all depend on satellites in Earth orbit. Or call your friends on Mauritius, Madagascar, or Maui: A satellite will relay your voice. Worried about the ozone hole over Antarctica or mass graves in Bosnia? Orbital outposts are keeping watch. The challenge these days is finding something that doesn't involve satellites in one way or other. And satellites are just one perk of the Space Age. Farther afield, robotic space probes have examined all the planets except Pluto, leading to a revolution in the Earth sciences -- from studies of plate tectonics to models of global warming -- now that scientists can compare our world to its planetary siblings. Over 300 people from 26 countries have gone into space, including the 24 astronauts who went on or near the Moon. Who knows how many will go in the next hundred years? In short, space travel has become a part of our lives. But what goes on behind the scenes? It turns out that satellites and spaceships depend on some of the most basic concepts of physics. So space travel isn't just fun to think about; it is a firm grounding in many of the principles that govern our world and our universe. -

Magnetoshell Aerocapture: Advances Toward Concept Feasibility
Magnetoshell Aerocapture: Advances Toward Concept Feasibility Charles L. Kelly A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Aeronautics & Astronautics University of Washington 2018 Committee: Uri Shumlak, Chair Justin Little Program Authorized to Offer Degree: Aeronautics & Astronautics c Copyright 2018 Charles L. Kelly University of Washington Abstract Magnetoshell Aerocapture: Advances Toward Concept Feasibility Charles L. Kelly Chair of the Supervisory Committee: Professor Uri Shumlak Aeronautics & Astronautics Magnetoshell Aerocapture (MAC) is a novel technology that proposes to use drag on a dipole plasma in planetary atmospheres as an orbit insertion technique. It aims to augment the benefits of traditional aerocapture by trapping particles over a much larger area than physical structures can reach. This enables aerocapture at higher altitudes, greatly reducing the heat load and dynamic pressure on spacecraft surfaces. The technology is in its early stages of development, and has yet to demonstrate feasibility in an orbit-representative envi- ronment. The lack of a proof-of-concept stems mainly from the unavailability of large-scale, high-velocity test facilities that can accurately simulate the aerocapture environment. In this thesis, several avenues are identified that can bring MAC closer to a successful demonstration of concept feasibility. A custom orbit code that dynamically couples magnetoshell physics with trajectory prop- agation is developed and benchmarked. The code is used to simulate MAC maneuvers for a 60 ton payload at Mars and a 1 ton payload at Neptune, both proposed NASA mis- sions that are not possible with modern flight-ready technology. In both simulations, MAC successfully completes the maneuver and is shown to produce low dynamic pressures and continuously-variable drag characteristics. -

Orbital Fueling Architectures Leveraging Commercial Launch Vehicles for More Affordable Human Exploration
ORBITAL FUELING ARCHITECTURES LEVERAGING COMMERCIAL LAUNCH VEHICLES FOR MORE AFFORDABLE HUMAN EXPLORATION by DANIEL J TIFFIN Submitted in partial fulfillment of the requirements for the degree of: Master of Science Department of Mechanical and Aerospace Engineering CASE WESTERN RESERVE UNIVERSITY January, 2020 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis of DANIEL JOSEPH TIFFIN Candidate for the degree of Master of Science*. Committee Chair Paul Barnhart, PhD Committee Member Sunniva Collins, PhD Committee Member Yasuhiro Kamotani, PhD Date of Defense 21 November, 2019 *We also certify that written approval has been obtained for any proprietary material contained therein. 2 Table of Contents List of Tables................................................................................................................... 5 List of Figures ................................................................................................................. 6 List of Abbreviations ....................................................................................................... 8 1. Introduction and Background.................................................................................. 14 1.1 Human Exploration Campaigns ....................................................................... 21 1.1.1. Previous Mars Architectures ..................................................................... 21 1.1.2. Latest Mars Architecture ......................................................................... -

Reusable Rocket Upper Stage Development of a Multidisciplinary Design Optimisation Tool to Determine the Feasibility of Upper Stage Reusability L
Reusable Rocket Upper Stage Development of a Multidisciplinary Design Optimisation Tool to Determine the Feasibility of Upper Stage Reusability L. Pepermans Technische Universiteit Delft Reusable Rocket Upper Stage Development of a Multidisciplinary Design Optimisation Tool to Determine the Feasibility of Upper Stage Reusability by L. Pepermans to obtain the degree of Master of Science at the Delft University of Technology, to be defended publicly on Wednesday October 30, 2019 at 14:30 AM. Student number: 4144538 Project duration: September 1, 2018 – October 30, 2019 Thesis committee: Ir. B.T.C Zandbergen , TU Delft, supervisor Prof. E.K.A Gill, TU Delft Dr.ir. D. Dirkx, TU Delft This thesis is confidential and cannot be made public until October 30, 2019. An electronic version of this thesis is available at http://repository.tudelft.nl/. Cover image: S-IVB upper stage of Skylab 3 mission in orbit [23] Preface Before you lies my thesis to graduate from Delft University of Technology on the feasibility and cost-effectiveness of reusable upper stages. During the accompanying literature study, it was determined that the technology readiness level is sufficiently high for upper stage reusability. However, it was unsure whether a cost-effective system could be build. I have been interested in the field of Entry, Descent, and Landing ever since I joined the Capsule Team of Delft Aerospace Rocket Engineering (DARE). During my time within the team, it split up in the Structures Team and Recovery Team. In September 2016, I became Chief Recovery for the Stratos III student-built sounding rocket. During this time, I realised that there was a lack of fundamental knowledge in aerodynamic decelerators within DARE. -

Smallsat Aerocapture to Enable a New Paradigm of Planetary Missions
SmallSat Aerocapture to Enable a New Paradigm of Planetary Missions Alex Austin, Adam Nelessen, Bill Ethiraj Venkatapathy, Robin Beck, Robert Braun, Michael Werner, Evan Strauss, Joshua Ravich, Mark Jesick Paul Wercinski, Michael Aftosmis, Roelke Jet Propulsion Laboratory, California Michael Wilder, Gary Allen CU Boulder Institute of Technology NASA Ames Research Center Boulder, CO 80309 Pasadena, CA 91109 Moffett Field, CA 94035 [email protected] 818-393-7521 [email protected] [email protected] Abstract— This paper presents a technology development system requirements, which concluded that mature TPS initiative focused on delivering SmallSats to orbit a variety of materials are adequate for this mission. CFD simulations were bodies using aerocapture. Aerocapture uses the drag of a single used to assess the risk of recontact by the drag skirt during the pass through the atmosphere to capture into orbit instead of jettison event. relying on large quantities of rocket fuel. Using drag modulation flight control, an aerocapture vehicle adjusts its drag area This study has concluded that aerocapture for SmallSats could during atmospheric flight through a single-stage jettison of a be a viable way to increase the delivered mass to Venus and can drag skirt, allowing it to target a particular science orbit in the also be used at other destinations. With increasing interest in presence of atmospheric uncertainties. A team from JPL, NASA SmallSats and the challenges associated with performing orbit Ames, and CU Boulder has worked to address the key insertion burns on small platforms, this technology could enable challenges and determine the feasibility of an aerocapture a new paradigm of planetary science missions. -

Aas 19-725 Improved Atmospheric Estimation for Aerocapture Guidance
AAS 19-725 IMPROVED ATMOSPHERIC ESTIMATION FOR AEROCAPTURE GUIDANCE Evan Roelke,∗ Phil D. Hattis,y and R.D. Braunz Increased interest in Lunar or Mars-sample return missions encourages considera- tion of innovative orbital operations such as aerocapture, which generally provides significant mass-savings for orbital insertion at Earth or Mars. Drag modulation architectures offer a straightforward approach to orbital apoapsis targeting by en- abling ballistic entry, among other benefits. A shortcoming of these architectures is the poor estimation of atmospheric density resulting in target apoapsis altitude errors. This research seeks to assess and improve upon current atmospheric den- sity estimation techniques in order to support the flight viability of discrete event drag modulated aerocapture. Three different estimation techniques are assessed in terms of estimation error and apoapsis altitude error: a static density factor, a density array interpolator, and an ensemble correlation filter. The density inter- polator achieves a 5% improvement in median apoapsis altitude over the density factor when entering at −5:9◦ and targeting a 2000km apoapsis altitude, while the ensemble correlation filter achieves a 7% improvement under identical simulation conditions. The ensemble correlation filter was found to improve with decreasing density search tolerance, achieving a 4:6% improvement in median apoapsis alti- tude for a tolerance of 1% over 5%. These improvements are dependent on entry and vehicle parameters and improve as the entry angle becomes more shallow or the target apoapsis is reduced. Errors in the density factor measurements are main contributors to the error in estimated versus true density profiles. INTRODUCTION The revitalized interest in Lunar missions as well as the drive for Mars sample-return missions in recent years encourages innovative solutions to orbital operations. -

Study of a Crew Transfer Vehicle Using Aerocapture for Cycler Based Exploration of Mars by Larissa Balestrero Machado a Thesis S
Study of a Crew Transfer Vehicle Using Aerocapture for Cycler Based Exploration of Mars by Larissa Balestrero Machado A thesis submitted to the College of Engineering and Science of Florida Institute of Technology in partial fulfillment of the requirements for the degree of Master of Science in Aerospace Engineering Melbourne, Florida May, 2019 © Copyright 2019 Larissa Balestrero Machado. All Rights Reserved The author grants permission to make single copies ____________________ We the undersigned committee hereby approve the attached thesis, “Study of a Crew Transfer Vehicle Using Aerocapture for Cycler Based Exploration of Mars,” by Larissa Balestrero Machado. _________________________________________________ Markus Wilde, PhD Assistant Professor Department of Aerospace, Physics and Space Sciences _________________________________________________ Andrew Aldrin, PhD Associate Professor School of Arts and Communication _________________________________________________ Brian Kaplinger, PhD Assistant Professor Department of Aerospace, Physics and Space Sciences _________________________________________________ Daniel Batcheldor Professor and Head Department of Aerospace, Physics and Space Sciences Abstract Title: Study of a Crew Transfer Vehicle Using Aerocapture for Cycler Based Exploration of Mars Author: Larissa Balestrero Machado Advisor: Markus Wilde, PhD This thesis presents the results of a conceptual design and aerocapture analysis for a Crew Transfer Vehicle (CTV) designed to carry humans between Earth or Mars and a spacecraft on an Earth-Mars cycler trajectory. The thesis outlines a parametric design model for the Crew Transfer Vehicle and presents concepts for the integration of aerocapture maneuvers within a sustainable cycler architecture. The parametric design study is focused on reducing propellant demand and thus the overall mass of the system and cost of the mission. This is accomplished by using a combination of propulsive and aerodynamic braking for insertion into a low Mars orbit and into a low Earth orbit. -

Author's Instructions For
Feasibility Analysis for a Manned Mars Free-Return Mission in 2018 Dennis A. Tito Grant Anderson John P. Carrico, Jr. Wilshire Associates Incorporated Paragon Space Development Applied Defense Solutions, Inc. 1800 Alta Mura Road Corporation 10440 Little Patuxent Pkwy Pacific Palisades, CA 90272 3481 East Michigan Street Ste 600 310-260-6600 Tucson, AZ 85714 Columbia, MD 21044 [email protected] 520-382-4812 410-715-0005 [email protected] [email protected] Jonathan Clark, MD Barry Finger Gary A Lantz Center for Space Medicine Paragon Space Development Paragon Space Development Baylor College Of Medicine Corporation Corporation 6500 Main Street, Suite 910 1120 NASA Parkway, Ste 505 1120 NASA Parkway, Ste 505 Houston, TX 77030-1402 Houston, TX 77058 Houston, TX 77058 [email protected] 281-702-6768 281-957-9173 ext #4618 [email protected] [email protected] Michel E. Loucks Taber MacCallum Jane Poynter Space Exploration Engineering Co. Paragon Space Development Paragon Space Development 687 Chinook Way Corporation Corporation Friday Harbor, WA 98250 3481 East Michigan Street 3481 East Michigan Street 360-378-7168 Tucson, AZ 85714 Tucson, AZ 85714 [email protected] 520-382-4815 520-382-4811 [email protected] [email protected] Thomas H. Squire S. Pete Worden Thermal Protection Materials Brig. Gen., USAF, Ret. NASA Ames Research Center NASA AMES Research Center Mail Stop 234-1 MS 200-1A Moffett Field, CA 94035-0001 Moffett Field, CA 94035 (650) 604-1113 650-604-5111 [email protected] [email protected] Abstract—In 1998 Patel et al searched for Earth-Mars free- To size the Environmental Control and Life Support System return trajectories that leave Earth, fly by Mars, and return to (ECLSS) we set the initial mission assumption to two crew Earth without any deterministic maneuvers after Trans-Mars members for 500 days in a modified SpaceX Dragon class of Injection. -

ARTIFICIAL GRAVITY RESEARCH to ENABLE HUMAN SPACE EXPLORATION International Academy of Astronautics
ARTIFICIAL GRAVITY RESEARCH TO ENABLE HUMAN SPACE EXPLORATION International Academy of Astronautics Notice: The cosmic study or position paper that is the subject of this report was approved by the Board of Trustees of the International Academy of Astronautics (IAA). Any opinions, findings, conclusions, or recommendations expressed in this report are those of the authors and do not necessarily reflect the views of the sponsoring or funding organizations. For more information about the International Academy of Astronautics, visit the IAA home page at www.iaaweb.org. Copyright 2009 by the International Academy of Astronautics. All rights reserved. The International Academy of Astronautics (IAA), a non governmental organization recognized by the United Nations, was founded in 1960. The purposes of the IAA are to foster the development of astronautics for peaceful purposes, to recognize individuals who have distinguished themselves in areas related to astronautics, and to provide a program through which the membership can contribute to international endeavors and cooperation in the advancement of aerospace activities. © International Academy of Astronautics (IAA) September 2009 Study on ARTIFICIAL GRAVITY RESEARCH TO ENABLE HUMAN SPACE EXPLORATION Edited by Laurence Young, Kazuyoshi Yajima and William Paloski Printing of this Study was sponsored by: DLR Institute of Aerospace Medicine, Cologne, Germany Linder Hoehe D-51147 Cologne, Germany www.dlr.de/me International Academy of Astronautics 6 rue Galilée, BP 1268-16, 75766 Paris Cedex 16, -

International Student Design Competition for Inspiration Mars Mission Report Summary (Team Kanau)
International Student Design Competition for Inspiration Mars Mission Report Summary (Team Kanau) Shota Iino1, Kshitij Mall2, Ayako Ono3, Jeff Stuart2, Ashwati Das2, Eriko Moriyama4, Takuya Ohgi5, Nick Gillin6, Koki Tanaka1, Yuri Aida1, Max Fagin2, Daichi Nakajima7 Professor Hiroyuki Miyajima8, Assistant Professor Michael Grant2 1Keio University (Japan), 2Purdue University (USA), 3Tohoku University Graduate School of Medicine (Alumnus, Japan), 4International Space University (Alumnus, France), 5Nagoya University (Alumnus, Japan), 6Art Center College of Design (USA), 7Tokyo University of Agriculture and Technology (Japan), 8Tokyo Jogakkan College (Japan). TEAM KANAU Table of Contents Abstract 1 1. Mission Objectives 1 2. Mission Design 1 2.1 Mission Design Methods 1 2.2 Requirements Generation 2 2.3 Concept Generation 8 2.4 Concept Selection 9 3. Concept of Operations 9 4. Trajectory Design and Launch Vehicle Selection 11 4.1 Overview 11 4.2 Interplanetary Ballistic Free-Return Trajectory 11 4.3 Earth Launch to LEO 13 5. Aerocapture 16 5.1 Sample Analysis 16 5.2 Results 17 6. Key Subsystem Architecture 18 6.1 Environment Control and Life Support System 18 6.2 Kanau Spacecraft’s Interior Design 24 6.3 Facilities for Crew Physical Health 26 6.4 Facilities for Radiation Protection 27 6.5 Command and Data Handling 27 6.6 Communications 29 6.7 Power Systems 31 6.8 Thermal Control System 36 6.9 Payload Mission 37 7. Safety Analysis and Design 38 7.1 Safety Requirements 38 7.2 Abort Options 39 7.3 Risk Acceptability 39 7.4 Risk Assessment 41 8. Crew Selection from US Astronauts 41 9. -

Aerocapture Technology Development Overview
Aerocapture Technology Development Overview Michelle M. Munk Mail Stop 494 NASA-Langley Research Center Hampton, VA 23681 757-864-2314 [email protected] Steven A. Moon Gray Research, Inc. Huntsville, AL 35806 256-922-9952 [email protected] Abstract—This paper will explain the investment strategy, ISPT portfolio is Aerocapture, which is the process of using the role of detailed systems analysis, and the hardware and a body’s atmosphere to slow an incoming spacecraft and modeling developments that have resulted from the past 5 place it into a useful science orbit (Fig. 1). years of work under NASA’s In-Space Propulsion Program (ISPT) Aerocapture investment area. The organizations that Entry targeting burn have been funded by ISPT over that time period received Atmospheric entry awards from a 2002 NASA Research Announcement. They are: Lockheed Martin Space Systems, Applied Research Energy Associates, Inc., Ball Aerospace, NASA’s Ames Research dissipation Center, and NASA’s Langley Research Center. Their Periapsis accomplishments include improved understanding of entry raise aerothermal environments, particularly at Titan, maneuver demonstration of aerocapture guidance algorithm robustness (propulsive Target orbit at multiple bodies, manufacture and test of a 2-meter Carbon-Carbon “hot structure,” development and test of Controlled exit evolutionary, high-temperature structural systems with efficient ablative materials, and development of aerothermal Jettison Aeroshell sensors that will fly on the Mars Science Laboratory in 2009. Due in large part to this sustained ISPT support for Figure 1 - Aerocapture Maneuver Aerocapture, the technology is ready to be validated in Aerocapture differs from aerobraking, a flight-proven flight.