Dipeptidyl Peptidase-4 and the Treatment of Type 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Semaglutide Versus Liraglutide for Treatment of Obesity
Archives of Diabetes & Obesity DOI: 10.32474/ADO.2021.03.000162 ISSN: 2638-5910 Review Article Semaglutide versus liraglutide for treatment of obesity Nasser Mikhail* *Department of Medicine, Endocrinology Division, David-Geffen UCLA Medical School, USA *Corresponding author: Nasser Mikhail, Endocrinology Division, Department of Medicine, Olive View-UCLA Medical Center, David- Geffen UCLA Medical School, CA, USA Received: April 02, 2021 Published: April 19, 2021 Abstract Background: Once weekly (OW) semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) currently under evaluation for treatment of obesity at a dose of 2.4 mg OW. Objective Methods : To compare weight-loss efficacy and safety of once daily (OD) liraglutide 3.0 mg versus OW semaglutide 2.4 mg. : Pubmed research up to March 31, 2021. Randomized trials, pertinent animal studies, and reviews are included. Search Results terms were glucagon-like peptide-1 receptor agonists, weight loss, obesity, liraglutide, semaglutide, efficacy, safety. semaglutide 2.4 mg. However, marked resemblance between trials in terms of study protocols and subjects’ characteristics may allow indirect: No comparison. head to head In clinical trials are trials available of OW tosemaglutide, provide direct this comparison drug was consistently of efficacy ofassociated OD liraglutide with greater 3.0 mg weightversus lossOW than in trials of OD liraglutide. Thus, placebo-corrected percentage weight reduction was -10.3 to -12.4% and -5.4% with OW semaglutide and OD liraglutide, respectively. In patients with type 2 diabetes, corresponding weight reduction was less pronounced with both drugs being -6.2% and -4.3% with OW semaglutide and OD liraglutide, respectively. -

A 26-Week Randomized Controlled Trial of Semaglutide Once Daily Versus Liraglutide and Placebo in Patients with Type 2 Diabetes
1926 Diabetes Care Volume 41, September 2018 Ildiko Lingvay,1 Cyrus V. Desouza,2 A 26-Week Randomized Katarina S. Lalic,3 Ludger Rose,4 Thomas Hansen,5 Jeppe Zacho,5 and Controlled Trial of Semaglutide Thomas R. Pieber6 EMERGING THERAPIES: DRUGS AND REGIMENS Once Daily Versus Liraglutide and Placebo in Patients With Type 2 Diabetes Suboptimally Controlled on Diet and Exercise With or Without Metformin Diabetes Care 2018;41:1926–1937 | https://doi.org/10.2337/dc17-2381 OBJECTIVE To investigate the efficacy and safety of once-daily semaglutide in comparison with once-daily liraglutide and placebo in patients with type 2 diabetes. RESEARCH DESIGN AND METHODS This 26-week, multicenter, double-blind trial involved patients diagnosed with 1University of Texas Southwestern Medical Cen- type 2 diabetes with HbA1c 7.0–10.0% (53–86 mmol/mol) and treated with diet ter at Dallas, Dallas, TX and exercise with or without metformin. Patients were randomized 2:2:1 to once- 2University of Nebraska Medical Center, Omaha, daily semaglutide, liraglutide, or placebo in one of four volume-matched doses NE 3Faculty of Medicine, University of Belgrade, and (semaglutide 0.05, 0.1, 0.2, or 0.3 mg and liraglutide 0.3, 0.6, 1.2, or 1.8 mg, with Clinic for Endocrinology, Diabetes and Metabolic both compared within each volume-matched dose group). Primary end point was Diseases, Clinical Center of Serbia, Belgrade, Serbia change in HbA1c from baseline to week 26. 4Institute for Diabetes Research in Munster,¨ RESULTS Munster,¨ Germany 5Novo Nordisk A/S, Søborg, Denmark In total, 705 randomized patients were exposed to trial products. -

Pharmacokinetics of Omarigliptin, a Once-Weekly Dipeptidyl Peptidase-4 Inhibitor
Available online a t www.derpharmachemica.com ISSN 0975-413X Der Pharma Chemica, 2016, 8(12):292-295 CODEN (USA): PCHHAX (http://derpharmachemica.com/archive.html) Mini-review: Pharmacokinetics of Omarigliptin, a Once-weekly Dipeptidyl Peptidase-4 Inhibitor Nermeen Ashoush a,b aClinical Pharmacy and Pharmacy Practice Department, Faculty of Pharmacy, British University in Egypt, El- Sherouk city, Cairo 11837, Egypt. bHead of Health Economics Unit, Center for Drug Research and Development (CDRD), Faculty of Pharmacy, British University in Egypt, El-Sherouk city, Cairo 11837, Egypt. _____________________________________________________________________________________________ ABSTRACT The dipeptidyl peptidase-4 (DPP-4) inhibitors are novel oral hypoglycemic drugs which have been in clinical use for the past 10 years. The drugs are safe, weight neutral and widely prescribed. There are currently many gliptins approved by FDA, namely sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin with several more in advanced stages of development. The gliptins may possess cardiovascular protective effects and their administration may promote β-cell survival; claims currently being evaluated in clinical and preclinical studies. The gliptins are an optional second-line therapy after metformin; they are generally well tolerated with low risk of hypoglycemia. The various compounds differ with respect to their pharmacokinetic properties; however, their clinical efficacy appears to be similar. The clinical differences between the various compounds -

An in Vivo Investigation Into the Actions of the Hypothalamic Neuropeptide, QRFP
An In Vivo Investigation into the Actions of the Hypothalamic Neuropeptide, QRFP A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Biology, Medicine and Health Christopher J Cook Faculty of Biology, Medicine and Health School of Medical Sciences 2017 Contents Abstract ........................................................................................................................................... 11 Declaration ........................................................................................................................................ 12 Copyright ........................................................................................................................................... 12 Acknowledgement ............................................................................................................................ 13 Chapter 1 Introduction ................................................................................................. 14 1.1 Energy homeostasis ................................................................................................................ 15 1.2 The control of food intake ...................................................................................................... 16 1.2.1 Peripheral signals regulating food intake .......................................................................... 17 1.2.2 Central aspects of food intake regulation ........................................................................ -

Safety and Efficacy of Omarigliptin (MK-3102), a Novel Once-Weekly
2106 Diabetes Care Volume 38, November 2015 fi Wayne H.-H. Sheu,1 Ira Gantz,2 Safety and Ef cacy of Omarigliptin Menghui Chen,2 Shailaja Suryawanshi,2 Arpana Mirza,2 Barry J. Goldstein,2 (MK-3102), a Novel Once-Weekly Keith D. Kaufman,2 and Samuel S. Engel2 DPP-4 Inhibitor for the Treatment of Patients With Type 2 Diabetes Diabetes Care 2015;38:2106–2114 | DOI: 10.2337/dc15-0109 OBJECTIVE This study was conducted to determine the optimal dose of omarigliptin, a once- weekly (q.w.) dipeptidyl peptidase IV (DPP-4) inhibitor, for the treatment of patients with type 2 diabetes and evaluate the long-term safety of that dose. RESEARCH DESIGN AND METHODS In a multicenter, double-blind, 12-week, dose-range finding study, 685 oral antihy- perglycemic agent-na¨ıve or washed-out subjects with type 2 diabetes were random- ized to one of five once-weekly doses of omarigliptin (0.25 mg, 1 mg, 3 mg, 10 mg, or 25 mg) or placebo. The primary efficacy end point was change from baseline in HbA1c, and secondary end points were 2-h postmeal glucose (PMG) and fasting plasma glucose (FPG). Analysis included all patients who received at least one dose of the study medication. Subjects who completed the base study were eligible to enter a 66-week extension study. RESULTS Once-weekly treatment for 12 weeks with omarigliptin provided dose-related reduc- 1Division of Endocrinology and Metabolism, De- partment of Internal Medicine, Taichung Veterans EMERGING TECHNOLOGIES AND THERAPEUTICS tions in HbA , 2-h PMG, and FPG. -

A Plant-Based Meal Increases Gastrointestinal Hormones
nutrients Article A Plant-Based Meal Increases Gastrointestinal Hormones and Satiety More Than an Energy- and Macronutrient-Matched Processed-Meat Meal in T2D, Obese, and Healthy Men: A Three-Group Randomized Crossover Study Marta Klementova 1, Lenka Thieme 1 , Martin Haluzik 1, Renata Pavlovicova 1, Martin Hill 2, Terezie Pelikanova 1 and Hana Kahleova 1,3,* 1 Institute for Clinical and Experimental Medicine, 140 21 Prague, Czech Republic; [email protected] (M.K.); [email protected] (L.T.); [email protected] (M.H.); [email protected] (R.P.); [email protected] (T.P.) 2 Institute of Endocrinology, 113 94 Prague, Czech Republic; [email protected] 3 Physicians Committee for Responsible Medicine, Washington, DC 20016, USA * Correspondence: [email protected]; Tel.: +1-202-527-7379 Received: 6 December 2018; Accepted: 9 January 2019; Published: 12 January 2019 Abstract: Gastrointestinal hormones are involved in regulation of glucose metabolism and satiety. We tested the acute effect of meal composition on these hormones in three population groups. A randomized crossover design was used to examine the effects of two energy- and macronutrient-matched meals: a processed-meat and cheese (M-meal) and a vegan meal with tofu (V-meal) on gastrointestinal hormones, and satiety in men with type 2 diabetes (T2D, n = 20), obese men (O, n = 20), and healthy men (H, n = 20). Plasma concentrations of glucagon-like peptide -1 (GLP-1), amylin, and peptide YY (PYY) were determined at 0, 30, 60, 120 and 180 min. Visual analogue scale was used to assess satiety. We used repeated-measures Analysis of variance (ANOVA) for statistical analysis. -

Subcutaneous Semaglutide, Dulaglutide and Liraglutide 1.2Mg for the Treatment of Type
North Central London Joint Formulary Committee Factsheet Subcutaneous SEMAGLUTIDE▼ (Ozempic®), DULAGLUTIDE▼ (Trulicity®) and LIRAGLUTIDE 1.2mg (Victoza®) Treatment of Type 2 Diabetes Mellitus Start date: December 2019 Review date: August 2022 Document Control Date Version Action July 2016 1.0 New guideline August 2019 1.1 Subcutaneous semaglutide added as the preferred GLP-1 receptor agonist November 2019 1.2 Supply quantities added Agreed by NCL Shared Care Group: December 2019 FACTSHEET TO FACILITATE PRESCRIBING PLEASE NOTE THIS IS NOT A SHARED CARE GUIDELINE, NOR IS IT A FULL SUMMARY OF DRUG INFORMATION. ALWAYS REFER TO THE MOST RECENT BNF AND/OR SUMMARY OF PRODUCT CHARACTERISTICS. Disclaimer This Fact Sheet is registered at North Central London (NCL) Joint Formulary Committee (JFC) and is intended solely for use by healthcare professionals to aid the treatment of patients within NCL. However, this fact sheet is for guidance only, its interpretation and application remains the responsibility of the individual clinician. If in doubt, contact a senior colleague or expert. Clinicians are advised to refer to the manufacturer’s current prescribing information before treating individual patients. The authors and NCL JFC accept no liability for use of this information from this beyond its intended use. While we have tried to compile accurate information in this document, and to keep it updated in a timely manner, we cannot guarantee that it is fully complete and correct at all times. If you identify information within this document that is inaccurate, please report this to the [email protected]. If a patient is harmed as a consequence of following this document, please complete a local incident report and inform [email protected]. -

Amylin: Pharmacology, Physiology, and Clinical Potential
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2015 Amylin: Pharmacology, Physiology, and Clinical Potential Hay, Debbie L ; Chen, Steve ; Lutz, Thomas A ; Parkes, David G ; Roth, Jonathan D Abstract: Amylin is a pancreatic -cell hormone that produces effects in several different organ systems. Here, we review the literature in rodents and in humans on amylin research since its discovery as a hormone about 25 years ago. Amylin is a 37-amino-acid peptide that activates its specific receptors, which are multisubunit G protein-coupled receptors resulting from the coexpression of a core receptor protein with receptor activity-modifying proteins, resulting in multiple receptor subtypes. Amylin’s major role is as a glucoregulatory hormone, and it is an important regulator of energy metabolism in health and disease. Other amylin actions have also been reported, such as on the cardiovascular system or on bone. Amylin acts principally in the circumventricular organs of the central nervous system and functionally interacts with other metabolically active hormones such as cholecystokinin, leptin, and estradiol. The amylin-based peptide, pramlintide, is used clinically to treat type 1 and type 2 diabetes. Clinical studies in obesity have shown that amylin agonists could also be useful for weight loss, especially in combination with other agents. DOI: https://doi.org/10.1124/pr.115.010629 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-112571 Journal Article Published Version Originally published at: Hay, Debbie L; Chen, Steve; Lutz, Thomas A; Parkes, David G; Roth, Jonathan D (2015). -

Victoza® (Liraglutide) Injection LEADER: Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results
Novo Nordisk Victoza® (liraglutide) NDA 22341 S-027 Endocrinologic and Metabolic Drug Advisory Committee, June 20, 2017 Victoza® (liraglutide) injection LEADER: Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results NDA 22341 S-027 Briefing Document Endocrinologic and Metabolic Drug Advisory Committee June 20, 2017 Advisory Committee Briefing Materials: Available for Public Release Novo Nordisk Victoza® (liraglutide) NDA 22341 S-027 Endocrinologic and Metabolic Drug Advisory Committee, June 20, 2017 2 of 95 1 Executive summary Introduction Liraglutide is an analog with 97% homology to human glucagon-like peptide-1 (GLP-1) and acts as a GLP-1 receptor agonist (GLP-1 RA). Liraglutide (Victoza®) obtained marketing authorization in the United States (US) in 2010 and is currently approved in over 100 countries worldwide. In the US, Victoza® (for subcutaneous injection 1.2 mg and 1.8 mg once-daily) is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (T2DM) as mono- or combination therapy.1 The exposure to Victoza® in the post-marketing setting is extensive; as of 30 June 2016, the cumulative exposure was estimated to be greater than 6 million patient-years of exposure to Victoza®. The LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) was a cardiovascular outcomes trial designed and conducted to determine the effect and long-term safety of liraglutide versus placebo on cardiovascular outcomes as -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

Efficacy and Safety of Taspoglutide Versus Sitagliptin
Diabetes Ther (2012) 3:13 DOI 10.1007/s13300-012-0013-8 ORIGINAL RESEARCH Efficacy and Safety of Taspoglutide Versus Sitagliptin for Type 2 Diabetes Mellitus (T-Emerge 4 Trial) Richard M. Bergenstal • Adriana Forti • Jean-Louis Chiasson • Michael Woloschak • Mark Boldrin • Raffaella Balena To view enhanced content go to www.diabetestherapy-open.com Received: August 9, 2012 / Published online: November 9, 2012 Ó The Author(s) 2012. This article is published with open access at Springerlink.com ABSTRACT sitagliptin or placebo, as adjunct to metformin, in patients with inadequately Introduction: The efficacy and safety of controlled type 2 diabetes. taspoglutide, a long-acting human glucagon- Methods: In this randomized, double-blind, like peptide-1 analog, were compared with double-dummy, parallel-group trial, patients were randomized to taspoglutide 10 mg once For the T-emerge 4 Study Group. weekly (QW), 20 mg QW, 100 mg sitagliptin Study investigators listed in the Appendix. once daily (QD), or placebo for 24 weeks, ClinicalTrials.gov identifier: NCT00754988. followed by 28-week short-term and 104-week long-term extension periods. The primary R. M. Bergenstal (&) endpoint was change in glycosylated Park Nicollet International Diabetes Center, hemoglobin (HbA ) after 24 weeks. Minneapolis, MN 55416, USA 1c e-mail: [email protected] Results: In this study, 666 patients (baseline HbA , 7.96% [SD, 0.87]; fasting plasma glucose, A. Forti 1c Universidade Federal do Ceara´, Fortaleza, Brazil 9.61 mmol/L [2.56]; body weight, 92.4 kg [19.3]) were randomized to taspoglutide 10 mg J.-L. Chiasson Centre de Recherche, Centre Hospitalier de QW (n = 190), 20 mg QW (n = 198), l’Universite´ de Montre´al, Universite´ de Montre´al, 100 mg sitagliptin QD (n = 185), or placebo Montreal, QC H2Q 1T8, Canada (n = 93) for 24 weeks. -
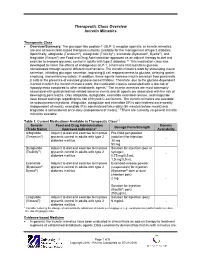
Therapeutic Class Overview Incretin Mimetics
Therapeutic Class Overview Incretin Mimetics Therapeutic Class Overview/Summary: The glucagon-like peptide-1 (GLP-1) receptor agonists, or incretin mimetics, are one of two incretin-based therapies currently available for the management of type 2 diabetes. Specifically, albiglutide (Tanzeum®), dulaglutide (Trulicity®), exenatide (Bydureon®, Byetta®), and liraglutide (Victoza®) are Food and Drug Administration-approved as an adjunct therapy to diet and exercise to improve glycemic control in adults with type 2 diabetes.1-5 This medication class was developed to mimic the effects of endogenous GLP-1, a hormone that maintains glucose homeostasis through several different mechanisms. The incretin mimetics work by stimulating insulin secretion, inhibiting glucagon secretion, improving β cell responsiveness to glucose, delaying gastric emptying, and enhancing satiety. In addition, these agents increase insulin secretion from pancreatic β cells in the presence of elevated glucose concentrations. Therefore, due to the glucose-dependent manner in which the incretin mimetics work, the medication class is associated with a low risk of hypoglycemia compared to other antidiabetic agents.6 The incretin mimetics are most commonly associated with gastrointestinal-related adverse events and all agents are associated with the risk of developing pancreatitis. Only albiglutide, dulaglutide, exenatide extended-release, and liraglutide have boxed warnings regarding the risk of thyroid C-cell tumors. The incretin mimetics are available as subcutaneous injections. Albiglutide, dulaglutide and exenatide ER is administered once-weekly (independent of meals), exenatide IR is administered twice-daily (60 minutes before meals) and liraglutide is administered once-daily (independent of meals).1-5 There are currently no generic incretin mimetics available. Table 1.