An in Vivo Investigation Into the Actions of the Hypothalamic Neuropeptide, QRFP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Kisspeptin-13 on the Hypothalamic-Pituitary-Adrenal Axis, Thermoregulation, Anxiety and Locomotor Activity in Rats
Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats Krisztina Csabafiª, Miklós Jászberényiª, Zsolt Bagosiª, Nándor Liptáka, Gyula Telegdyª,b a Department of Pathophysiology, University of Szeged, P.O. Box 427, H-6701Szeged, Hungary b Neuroscience Research Group of the Hungarian Academy of Sciences, P.O. Box 521, H- 6701Szeged, Hungary Corresponding author: Krisztina Csabafi MD Department of Pathophysiology, University of Szeged H-6701 Szeged, Semmelweis u. 1, PO Box: 427 Hungary Tel.:+ 36 62 545994 Fax: + 36 62 545710 E-mail: [email protected] 1 Abstract Kisspeptin is a mammalian amidated neurohormone, which belongs to the RF-amide peptide family and is known for its key role in reproduction. However, in contrast with the related members of the RF-amide family, little information is available regarding its role in the stress-response. With regard to the recent data suggesting kisspeptin neuronal projections to the paraventricular nucleus, in the present experiments we investigated the effect of kisspeptin-13 (KP-13), an endogenous derivative of kisspeptin, on the hypothalamus- pituitary-adrenal (HPA) axis, motor behavior and thermoregulatory function. The peptide was administered intracerebroventricularly (icv.) in different doses (0.5-2 µg) to adult male Sprague-Dawley rats, the behavior of which was then observed by means of telemetry, open field and elevated plus maze tests. Additionally, plasma concentrations of corticosterone were measured in order to assess the influence of KP-13 on the HPA system. The effects on core temperature were monitored continuously via telemetry. The results demonstrated that KP-13 stimulated the horizontal locomotion (square crossing) in the open field test and decreased the number of entries into and the time spent in the open arms during the elevated plus maze tests. -

Searching for Novel Peptide Hormones in the Human Genome Olivier Mirabeau
Searching for novel peptide hormones in the human genome Olivier Mirabeau To cite this version: Olivier Mirabeau. Searching for novel peptide hormones in the human genome. Life Sciences [q-bio]. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00340710 HAL Id: tel-00340710 https://tel.archives-ouvertes.fr/tel-00340710 Submitted on 21 Nov 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC THESE pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Discipline : Biologie Informatique Ecole Doctorale : Sciences chimiques et biologiques pour la santé Formation doctorale : Biologie-Santé Recherche de nouvelles hormones peptidiques codées par le génome humain par Olivier Mirabeau présentée et soutenue publiquement le 30 janvier 2008 JURY M. Hubert Vaudry Rapporteur M. Jean-Philippe Vert Rapporteur Mme Nadia Rosenthal Examinatrice M. Jean Martinez Président M. Olivier Gascuel Directeur M. Cornelius Gross Examinateur Résumé Résumé Cette thèse porte sur la découverte de gènes humains non caractérisés codant pour des précurseurs à hormones peptidiques. Les hormones peptidiques (PH) ont un rôle important dans la plupart des processus physiologiques du corps humain. -

Pharmacokinetics of Omarigliptin, a Once-Weekly Dipeptidyl Peptidase-4 Inhibitor
Available online a t www.derpharmachemica.com ISSN 0975-413X Der Pharma Chemica, 2016, 8(12):292-295 CODEN (USA): PCHHAX (http://derpharmachemica.com/archive.html) Mini-review: Pharmacokinetics of Omarigliptin, a Once-weekly Dipeptidyl Peptidase-4 Inhibitor Nermeen Ashoush a,b aClinical Pharmacy and Pharmacy Practice Department, Faculty of Pharmacy, British University in Egypt, El- Sherouk city, Cairo 11837, Egypt. bHead of Health Economics Unit, Center for Drug Research and Development (CDRD), Faculty of Pharmacy, British University in Egypt, El-Sherouk city, Cairo 11837, Egypt. _____________________________________________________________________________________________ ABSTRACT The dipeptidyl peptidase-4 (DPP-4) inhibitors are novel oral hypoglycemic drugs which have been in clinical use for the past 10 years. The drugs are safe, weight neutral and widely prescribed. There are currently many gliptins approved by FDA, namely sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin with several more in advanced stages of development. The gliptins may possess cardiovascular protective effects and their administration may promote β-cell survival; claims currently being evaluated in clinical and preclinical studies. The gliptins are an optional second-line therapy after metformin; they are generally well tolerated with low risk of hypoglycemia. The various compounds differ with respect to their pharmacokinetic properties; however, their clinical efficacy appears to be similar. The clinical differences between the various compounds -

Neuropeptide FF Increases M2 Activation and Self-Renewal of Adipose Tissue Macrophages
RESEARCH ARTICLE The Journal of Clinical Investigation Neuropeptide FF increases M2 activation and self-renewal of adipose tissue macrophages Syed F. Hassnain Waqas,1 Anh Cuong Hoang,1 Ya-Tin Lin,2 Grace Ampem,1 Hind Azegrouz,3 Lajos Balogh,4 Julianna Thuróczy,5 Jin-Chung Chen,2 Ivan C. Gerling,6 Sorim Nam,7 Jong-Seok Lim,7 Juncal Martinez-Ibañez,8 José T. Real,8 Stephan Paschke,9 Raphaëlle Quillet,10 Safia Ayachi,10 Frédéric Simonin,10 E. Marion Schneider,11 Jacqueline A. Brinkman,12,13 Dudley W. Lamming,12,13 Christine M. Seroogy,12 and Tamás Röszer 1 1Institute of Comparative Molecular Endocrinology, University of Ulm, Ulm, Germany. 2Department of Physiology and Pharmacology and Graduate Institute of Biomedical Sciences, Chang Gung University; Neuroscience Research Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan. 3Massachusetts Institute of Technology, Cambridge, Massachusetts, USA. 4National Research Institute for Radiobiology and Radiohygiene, Budapest, Hungary. 5University of Veterinary Medicine, Budapest, Hungary. 6Department of Medicine, University of Tennessee, Memphis, Tennessee, USA. 7Department of Biological Science, Sookmyung Women’s University, Seoul, South Korea. 8Department of Medicine, Hospital Clínico Universitario de València, Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Valencia, Spain. 9Department of General and Visceral Surgery, University Hospital Ulm, Ulm, Germany. 10Biotechnologie et Signalisation Cellulaire, UMR 7242, Centre National de Recherche Scientifique (CNRS), Université de Strasbourg, Illkirch, France. 11Division of Experimental Anesthesiology, University Hospital Ulm, Ulm, Germany. 12University of Wisconsin, School of Medicine and Public Health, Madison, Wisconsin, USA. 13William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA. The quantity and activation state of adipose tissue macrophages (ATMs) impact the development of obesity-induced metabolic diseases. -

Involvement of the Glutamine RF‑Amide Peptide and Its Cognate Receptor GPR103 in Prostate Cancer
1140 ONCOLOGY REPORTS 41: 1140-1150, 2019 Involvement of the glutamine RF‑amide peptide and its cognate receptor GPR103 in prostate cancer MOHAMED AB. KAWAN1*, IOANNIS KYROU1-4*, Manjunath Ramanjaneya1, KEVIN WILLIAMS5, Jeyarooban Jeyaneethi6, Harpal S. Randeva1-4** and EMMANOUIL Karteris6** 1Translational and Experimental Medicine, Clinical Sciences Research Institute, Warwick Medical School, University of Warwick, Coventry CV4 7AL; 2Warwickshire Institute for The Study of Diabetes, Endocrinology and Metabolism (WISDEM), University Hospitals Coventry and Warwickshire NHS Trust, Coventry CV2 2DX; 3Aston Medical Research Institute, Aston Medical School, Aston University, Birmingham B4 7ET; 4Centre of Applied Biological and Exercise Sciences, Faculty of Health and Life Sciences, Coventry University, Coventry CV1 5FB; 5Department of Urology, University Hospitals Coventry and Warwickshire NHS Trust, Coventry CV2 2DX; 6Biosciences, Department of Life Sciences, Brunel University, Uxbridge, Middlesex UB8 3PH, UK Received January 24, 2018; Accepted July 10, 2018 DOI: 10.3892/or.2018.6893 Abstract. Glutamine RF-amide peptide (QRFP) belongs to human prostate cancer cell lines (PC3 and DU145) as in vitro the RFamide neuropeptide family, which is involved in a wide experimental models and clinical human prostate cancer spectrum of biological activities, ranging from food intake and samples. The expression of both QRFP and GPR103 at the gene cardiovascular functioning to analgesia, aldosterone secre- and protein level was higher in human prostate cancer tissue tion, locomotor activity and reproduction. Recently, QRFP samples compared to control and benign prostatic hyperplasia has been demonstrated to exert its effects by activating the (BHP) samples. Furthermore, in both prostate cancer cell lines G protein-coupled receptor GPR103. QRFP is expressed in the used in the present study, QRFP treatment induced the phos- brain and peripherally in the adipose tissue, bladder, colon, phorylation of ERK1/2, p38, JNK and Akt. -

Safety and Efficacy of Omarigliptin (MK-3102), a Novel Once-Weekly
2106 Diabetes Care Volume 38, November 2015 fi Wayne H.-H. Sheu,1 Ira Gantz,2 Safety and Ef cacy of Omarigliptin Menghui Chen,2 Shailaja Suryawanshi,2 Arpana Mirza,2 Barry J. Goldstein,2 (MK-3102), a Novel Once-Weekly Keith D. Kaufman,2 and Samuel S. Engel2 DPP-4 Inhibitor for the Treatment of Patients With Type 2 Diabetes Diabetes Care 2015;38:2106–2114 | DOI: 10.2337/dc15-0109 OBJECTIVE This study was conducted to determine the optimal dose of omarigliptin, a once- weekly (q.w.) dipeptidyl peptidase IV (DPP-4) inhibitor, for the treatment of patients with type 2 diabetes and evaluate the long-term safety of that dose. RESEARCH DESIGN AND METHODS In a multicenter, double-blind, 12-week, dose-range finding study, 685 oral antihy- perglycemic agent-na¨ıve or washed-out subjects with type 2 diabetes were random- ized to one of five once-weekly doses of omarigliptin (0.25 mg, 1 mg, 3 mg, 10 mg, or 25 mg) or placebo. The primary efficacy end point was change from baseline in HbA1c, and secondary end points were 2-h postmeal glucose (PMG) and fasting plasma glucose (FPG). Analysis included all patients who received at least one dose of the study medication. Subjects who completed the base study were eligible to enter a 66-week extension study. RESULTS Once-weekly treatment for 12 weeks with omarigliptin provided dose-related reduc- 1Division of Endocrinology and Metabolism, De- partment of Internal Medicine, Taichung Veterans EMERGING TECHNOLOGIES AND THERAPEUTICS tions in HbA , 2-h PMG, and FPG. -

Evolution of Neuropeptide Signalling Systems (Doi:10.1242/Jeb.151092) Maurice R
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb193342. doi:10.1242/jeb.193342 CORRECTION Correction: Evolution of neuropeptide signalling systems (doi:10.1242/jeb.151092) Maurice R. Elphick, Olivier Mirabeau and Dan Larhammar There was an error published in J. Exp. Biol. (2018) 221, jeb151092 (doi:10.1242/jeb.151092). In Fig. 2, panels B and C are identical. The correct figure is below. The authors apologise for any inconvenience this may have caused. Journal of Experimental Biology 1 © 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb151092. doi:10.1242/jeb.151092 REVIEW Evolution of neuropeptide signalling systems Maurice R. Elphick1,*,‡, Olivier Mirabeau2,* and Dan Larhammar3,* ABSTRACT molecular to the behavioural level (Burbach, 2011; Schoofs et al., Neuropeptides are a diverse class of neuronal signalling molecules 2017; Taghert and Nitabach, 2012; van den Pol, 2012). that regulate physiological processes and behaviour in animals. Among the first neuropeptides to be chemically identified in However, determining the relationships and evolutionary origins of mammals were the hypothalamic neuropeptides vasopressin and the heterogeneous assemblage of neuropeptides identified in a range oxytocin, which act systemically as hormones (e.g. regulating of phyla has presented a huge challenge for comparative physiologists. diuresis and lactation) and act within the brain to influence social Here, we review revolutionary insights into the evolution of behaviour (Donaldson and Young, 2008; Young et al., 2011). neuropeptide signalling that have been obtained recently through Evidence of the evolutionary antiquity of neuropeptide signalling comparative analysis of genome/transcriptome sequence data and by emerged with the molecular identification of neuropeptides in – ‘deorphanisation’ of neuropeptide receptors. -
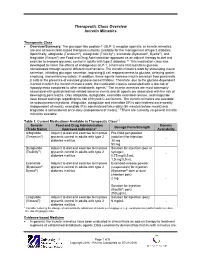
Therapeutic Class Overview Incretin Mimetics
Therapeutic Class Overview Incretin Mimetics Therapeutic Class Overview/Summary: The glucagon-like peptide-1 (GLP-1) receptor agonists, or incretin mimetics, are one of two incretin-based therapies currently available for the management of type 2 diabetes. Specifically, albiglutide (Tanzeum®), dulaglutide (Trulicity®), exenatide (Bydureon®, Byetta®), and liraglutide (Victoza®) are Food and Drug Administration-approved as an adjunct therapy to diet and exercise to improve glycemic control in adults with type 2 diabetes.1-5 This medication class was developed to mimic the effects of endogenous GLP-1, a hormone that maintains glucose homeostasis through several different mechanisms. The incretin mimetics work by stimulating insulin secretion, inhibiting glucagon secretion, improving β cell responsiveness to glucose, delaying gastric emptying, and enhancing satiety. In addition, these agents increase insulin secretion from pancreatic β cells in the presence of elevated glucose concentrations. Therefore, due to the glucose-dependent manner in which the incretin mimetics work, the medication class is associated with a low risk of hypoglycemia compared to other antidiabetic agents.6 The incretin mimetics are most commonly associated with gastrointestinal-related adverse events and all agents are associated with the risk of developing pancreatitis. Only albiglutide, dulaglutide, exenatide extended-release, and liraglutide have boxed warnings regarding the risk of thyroid C-cell tumors. The incretin mimetics are available as subcutaneous injections. Albiglutide, dulaglutide and exenatide ER is administered once-weekly (independent of meals), exenatide IR is administered twice-daily (60 minutes before meals) and liraglutide is administered once-daily (independent of meals).1-5 There are currently no generic incretin mimetics available. Table 1. -

Comparative Transcriptome Profiling of the Human and Mouse Dorsal Root
Research Paper Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq–based resource for pain and sensory neuroscience research a,b a a a a a 07/20/2018 on BhDMf5ePHKav1zEoum1tQfN4a+kJLhEZgbsIHo4XMi0hCywCX1AWnYQp/IlQrHD3mH5nK33R3Qh4f27oe7zFUUf7ZAUK5aCsyqAeT54jiDxP7ZjumT3TrA== by https://journals.lww.com/pain from Downloaded Pradipta Ray , Andrew Torck , Lilyana Quigley , Andi Wangzhou , Matthew Neiman , Chandranshu Rao , Downloaded Tiffany Lama, Ji-Young Kima, Tae Hoon Kimb, Michael Q. Zhangb, Gregory Dussora, Theodore J. Pricea,* from https://journals.lww.com/pain Abstract Molecular neurobiological insight into human nervous tissues is needed to generate next-generation therapeutics for neurological disorders such as chronic pain. We obtained human dorsal root ganglia (hDRG) samples from organ donors and performed RNA- sequencing (RNA-seq) to study the hDRG transcriptional landscape, systematically comparing it with publicly available data from by BhDMf5ePHKav1zEoum1tQfN4a+kJLhEZgbsIHo4XMi0hCywCX1AWnYQp/IlQrHD3mH5nK33R3Qh4f27oe7zFUUf7ZAUK5aCsyqAeT54jiDxP7ZjumT3TrA== a variety of human and orthologous mouse tissues, including mouse DRG (mDRG). We characterized the hDRG transcriptional profile in terms of tissue-restricted gene coexpression patterns and putative transcriptional regulators, and formulated an information-theoretic framework to quantify DRG enrichment. Relevant gene families and pathways were also analyzed, including transcription factors, G-protein-coupled receptors, and ion channels. Our analyses reveal an hDRG-enriched protein-coding gene set (;140), some of which have not been described in the context of DRG or pain signaling. Most of these show conserved enrichment in mDRG and were mined for known drug–gene product interactions. Conserved enrichment of the vast majority of transcription factors suggests that the mDRG is a faithful model system for studying hDRG, because of evolutionarily conserved regulatory programs. -

Genetic Identification of Brain Cell Types Underlying Schizophrenia
bioRxiv preprint doi: https://doi.org/10.1101/145466; this version posted June 2, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Genetic identification of brain cell types underlying schizophrenia Nathan G. Skene 1 †, Julien Bryois 2 †, Trygve E. Bakken3, Gerome Breen 4,5, James J Crowley 6, Héléna A Gaspar 4,5, Paola Giusti-Rodriguez 6, Rebecca D Hodge3, Jeremy A. Miller 3, Ana Muñoz-Manchado 1, Michael C O’Donovan 7, Michael J Owen 7, Antonio F Pardiñas 7, Jesper Ryge 8, James T R Walters 8, Sten Linnarsson 1, Ed S. Lein 3, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Patrick F Sullivan 2,6 *, Jens Hjerling- Leffler 1 * Affiliations: 1 Laboratory of Molecular Neurobiology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, SE-17177 Stockholm, Sweden. 2 Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, SE-17177 Stockholm, Sweden. 3 Allen Institute for Brain Science, Seattle, Washington 98109, USA. 4 King’s College London, Institute of Psychiatry, Psychology and Neuroscience, MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, London, UK. 5 National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, London, UK. 6 Departments of Genetics, University of North Carolina, Chapel Hill, NC, 27599-7264, USA. 7 MRC Centre for Neuropsychiatric Genetics and Genomics, Institute of Psychological Medicine and Clinical Neurosciences, School of Medicine, Cardiff University, Cardiff, UK. -

Rfamide Peptides: Structure, Function, Mechanisms and Pharmaceutical Potential
Pharmaceuticals 2011, 4, 1248-1280; doi:10.3390/ph4091248 OPEN ACCESS Pharmaceuticals ISSN 1424-8247 www.mdpi.com/journal/pharmaceuticals Review RFamide Peptides: Structure, Function, Mechanisms and Pharmaceutical Potential Maria Findeisen †, Daniel Rathmann † and Annette G. Beck-Sickinger * Institute of Biochemistry, Leipzig University, Brüderstraße 34, 04103 Leipzig, Germany; E-Mails: [email protected] (M.F.); [email protected] (D.R.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-341-9736900; Fax: +49-341-9736909. Received: 29 August 2011; in revised form: 9 September 2011 / Accepted: 15 September 2011 / Published: 21 September 2011 Abstract: Different neuropeptides, all containing a common carboxy-terminal RFamide sequence, have been characterized as ligands of the RFamide peptide receptor family. Currently, five subgroups have been characterized with respect to their N-terminal sequence and hence cover a wide pattern of biological functions, like important neuroendocrine, behavioral, sensory and automatic functions. The RFamide peptide receptor family represents a multiligand/multireceptor system, as many ligands are recognized by several GPCR subtypes within one family. Multireceptor systems are often susceptible to cross-reactions, as their numerous ligands are frequently closely related. In this review we focus on recent results in the field of structure-activity studies as well as mutational exploration of crucial positions within this GPCR system. The review summarizes the reported peptide analogs and recently developed small molecule ligands (agonists and antagonists) to highlight the current understanding of the pharmacophoric elements, required for affinity and activity at the receptor family. -

RF313, an Orally Bioavailable Neuropeptide FF Receptor
RF313, an orally bioavailable neuropeptide FF receptor antagonist, opposes effects of RF-amide-related peptide-3 and opioid-induced hyperalgesia in rodents Khadija Elhabazi, Jean-Paul Humbert, Isabelle Bertin, Raphaelle Quillet, Valérie Utard, Martine Schmitt, Jean-Jacques Bourguignon, Emilie Laboureyras, Meric Ben Boujema, Guy Simonnet, et al. To cite this version: Khadija Elhabazi, Jean-Paul Humbert, Isabelle Bertin, Raphaelle Quillet, Valérie Utard, et al.. RF313, an orally bioavailable neuropeptide FF receptor antagonist, opposes effects of RF-amide- related peptide-3 and opioid-induced hyperalgesia in rodents. Neuropsychopharmacology, Nature Publishing Group, 2017, 118, pp.188-198. 10.1016/j.neuropharm.2017.03.012. hal-01603852 HAL Id: hal-01603852 https://hal.archives-ouvertes.fr/hal-01603852 Submitted on 25 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Copyright Accepted Manuscript RF313, an orally bioavailable neuropeptide FF receptor antagonist, opposes effects of RF-amide-related peptide-3 and opioid-induced hyperalgesia in rodents