(12) Patent Application Publication (10) Pub. No.: US 2006/0018937 A1 Friedman Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Seco-Steroids C07C)
CPC - C07J - 2021.08 C07J STEROIDS (seco-steroids C07C) Definition statement This place covers: Compounds containing a cyclopenta[a]hydrophenanthrene skeleton (see below) or a ring structure derived therefrom: • by contraction or expansion of one ring by one or two atoms; • by contraction or expansion of two rings each by one atom; • by contraction of one ring by one atom and expansion of one ring by one atom; • by substitution of one or two carbon atoms of the cyclopenta[a]hydrophenanthrene skeleton, which are not shared by rings, by hetero atoms, in combination with the above defined contraction or expansion or not, or; • by condensation with carbocyclic or heterocyclic rings in combination with one or more of the foregoing alterations or not. Preparation of steroids including purification, separation, stabilisation or use of additives unless provided for elsewhere, as specified below. Treatment and modification of steroids provided that • the treatment is not provided for elsewhere and • the resultant product is a compound under the subclass definition. Relationships with other classification places In class C07, in the absence of an indication to the contrary, a compound is classified in the last appropriate place, i.e. in the last appropriate subclass. For example cyclopenta [a] hydrophenantrenes are classified in subclass C07J as steroids and not in subclasses C07C or C07D as carbocyclic or heterocyclic compounds. Subclass C07J is a function-oriented entry for the compounds themselves and does not cover the application or use of the compounds under the subclass definition. For classifying such information other entries in the IPC exist, for example: Subclass A01N: Preservation of bodies of humans or animals or plants or parts thereof; biocides, e.g. -

Albany-Molecular-Research-Regulatory
PRODUCT CATALOGUE API COMMERCIAL US EU Japan US EU Japan API Name Site CEP India API Name Site CEP India DMF DMF DMF DMF DMF DMF A Abiraterone Malta • Benztropine Mesylate Cedarburg • Adenosine Rozzano - Quinto de' Stampi • • * Betaine Citrate Anhydrous Bon Encontre • Betametasone-17,21- Alcaftadine Spain Spain • • Dipropionate Sterile • Alclometasone-17, 21- Spain Betamethasone Acetate Spain Dipropionate • • Altrenogest Spain • • Betamethasone Base Spain Amphetamine Aspartate Rensselaer Betamethasone Benzoate Spain * Monohydrate Milled • Betamethasone Valerate Amphetamine Sulfate Rensselaer Spain * • Acetate Betamethasone-17,21- Argatroban Rozzano - Quinto de' Stampi Spain • • Dipropionate • • • Atenolol India • • Betamethasone-17-Valerate Spain • • Betamethasone-21- Atracurium Besylate Rozzano - Quinto de' Stampi Spain • Phosphate Disodium Salt • • Bromfenac Monosodium Atropine Sulfate Cedarburg Lodi * • Salt Sesquihydrate • • Azanidazole Lodi Bromocriptine Mesylate Rozzano - Quinto de' Stampi • • • • • Azelastine HCl Rozzano - Quinto de' Stampi • • Budesonide Spain • • Aztreonam Rozzano - Valle Ambrosia • • Budesonide Sterile Spain • • B Bamifylline HCl Bon Encontre • Butorphanol Tartrate Cedarburg • Beclomethasone-17, 21- Spain Capecitabine Lodi Dipropionate • C • 2 *Please contact our Accounts Managers in case you are interested in this API. 3 PRODUCT CATALOGUE API COMMERCIAL US EU Japan US EU Japan API Name Site CEP India API Name Site CEP India DMF DMF DMF DMF DMF DMF Dexamethasone-17,21- Carbimazole Bon Encontre Spain • Dipropionate -

Role of Synthetic and Natural Inhibitors
Biochimica et Biophysica Acta 1845 (2014) 136–154 Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbacan Review Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors Kodappully Sivaraman Siveen a,1, Sakshi Sikka a,b,1,RohitSuranaa,b, Xiaoyun Dai a, Jingwen Zhang a, Alan Prem Kumar a,b,c,d, Benny K.H. Tan a, Gautam Sethi a,b,⁎, Anupam Bishayee e,⁎⁎ a Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore b Cancer Science Institute of Singapore, National University of Singapore, Centre for Translational Medicine, Singapore c School of Biomedical Sciences, Faculty of Health Sciences, Curtin University, Western Australia, Australia d Department of Biological Sciences, University of North Texas, Denton, TX, USA e Department of Pharmaceutical Sciences, School of Pharmacy, American University of Health Sciences, Signal Hill, CA, USA article info abstract Article history: Signal transducers and activators of transcription (STATs) comprise a family of cytoplasmic transcription factors Received 15 August 2013 that mediate intracellular signaling that is usually generated at cell surface receptors and thereby transmit it to Received in revised form 24 December 2013 the nucleus. Numerous studies have demonstrated constitutive activation of STAT3 in a wide variety of human Accepted 27 December 2013 tumors, including hematological malignancies (leukemias, lymphomas, and multiple myeloma) as well as Available online 2 January 2014 diverse solid tumors (such as head and neck, breast, lung, gastric, hepatocellular, colorectal and prostate cancers). There is strong evidence to suggest that aberrant STAT3 signaling promotes initiation and progression of human Keywords: STAT3 cancers by either inhibiting apoptosis or inducing cell proliferation, angiogenesis, invasion, and metastasis. -

Appendix on Tariff Elimination Schedule for Mercosur
Trade part of the EU-Mercosur Association Agreement Without Prejudice Disclaimer: In view of the Commission's transparency policy, the Commission is publishing the texts of the Trade Part of the Agreement following the agreement in principle announced on 28 June 2019. The texts are published for information purposes only and may undergo further modifications including as a result of the process of legal revision. However, in view of the growing public interest in the negotiations, the texts are published at this stage of the negotiations for information purposes. These texts are without prejudice to the final outcome of the agreement between the EU and Mercosur. The texts will be final upon signature. The agreement will become binding on the Parties under international law only after completion by each Party of its internal legal procedures necessary for the entry into force of the Agreement (or its provisional application). AR applied BR applied PY applied UY applied Mercosur Final NCM Description Comments tariff tariff tariff tariff Offer 01012100 Pure-bred horses 0 0 0 0 0 01012900 Lives horses, except pure-bred breeding 2 2 2 2 0 01013000 Asses, pure-bred breeding 4 4 4 4 4 01019000 Asses, except pure-bred breeding 4 4 4 4 4 01022110 Purebred breeding cattle, pregnant or lactating 0 0 0 0 0 01022190 Other pure-bred cattle, for breeding 0 0 0 0 0 01022911 Other bovine animals for breeding,pregnant or lactating 2 2 2 2 0 01022919 Other bovine animals for breeding 2 2 2 2 4 01022990 Other live catlle 2 2 2 2 0 01023110 Pure-bred breeding buffalo, pregnant or lactating 0 0 0 0 0 01023190 Other pure-bred breeding buffalo 0 0 0 0 0 01023911 Other buffalo for breeding, ex. -

Tibolone Has Anti-Inflammatory Effects in Estrogen-Deficient
Regulatory Peptides 179 (2012) 55–60 Contents lists available at SciVerse ScienceDirect Regulatory Peptides journal homepage: www.elsevier.com/locate/regpep Tibolone has anti-inflammatory effects in estrogen-deficient female rats on the natriuretic peptide system and TNF-alpha Ana Raquel Santos de Medeiros a,b, Aline Zandonadi Lamas b, Izabela Facco Caliman b, Polyana L. Meireles Dalpiaz b, Luciana Barbosa Firmes c, Gláucia Rodrigues de Abreu b, Margareth Ribeiro Moysés b, Elenice Moreira Lemos d, Adelina Martha dos Reis c, Nazaré Souza Bissoli b,⁎ a Biological and Health Sciences, Federal Institute of Espírito Santo, Vila Velha, ES, Brazil b Department of Physiological Sciences, Federal University of Espírito Santo, Vitória, ES, Brazil c Department of Physiology and Biophysics, Federal University of Minas Gerais, Belo Horizonte, MG, Brazil d Center of Infectious Diseases, Federal University of Espírito Santo, Vitória, ES, Brazil article i nfo abstract Article history: Cardiovascular and immune system abnormalities have been reported in females with estrogen deficiency. Received 25 April 2012 To control these disorders in post-menopausal women, hormone replacement therapy (HRT) has been Received in revised form 10 August 2012 used. Tibolone has been used as a HRT, but the effects of tibolone on the natriuretic peptide system have Accepted 29 August 2012 not been determined. We investigated the effects of tibolone on the natriuretic peptide system and Available online 10 September 2012 pro-inflammatory cytokines in ovariectomized (OVX) rats. Female rats were divided into four groups: SHAM, OVX, OVX treated with 17β-estradiol (OVX+E: 14 days) and OVX treated with tibolone (OVX+T: Keywords: Ovariectomy 14 days) beginning 21 days after ovariectomy. -

Efficiency of Different Methods of Estrus Synchronization Followed by Fixed Time Artificial Insemination in Persian Downy Does
DOI: 10.21451/1984-3143-AR825 Anim. Reprod., v.14, n.2, p.413-417, Apr./Jun. 2017 Efficiency of different methods of estrus synchronization followed by fixed time artificial insemination in Persian downy does Majid Hashemi1, 2, 3, Mazaher Safdarian2 1Razi Vaccine and Serum Research Institute, Shiraz Branch, Agricultural Research, Education and Extension Organization (AREEO), Shiraz, Iran. 2Animal Science Research Department, Fars Agricultural and Natural Resource Research and Education Center, Agricultural Research, Education and Extension Organization (AREEO), Shiraz, Iran. Abstract estrus synchronization can play an important role for managing production system, allowing the density of For evaluating different methods of long term estrous mating and kidding and production of meat and milk synchronization followed by fixed time artificial during specific times of the year for strategic marketing insemination and to select the most efficient method, and other purposes (Baldassarre and Karatzas, 2004, during the breeding season 160 Persian downy does Zhao et al., 2010). In small ruminants, hormonal estrus were equally allocated to groups (n = 20/group). Estrus synchronization is achieved either by reducing the was synchronized using controlled internal drug release length of the luteal phase of the estrous cycle with devices alone (CIDR) or with equine chorionic prostaglandin F2α or by extending the cycle artificially gonadotropin (CIDR-eCG), intravaginal sponge with exogenous progesterone or more potent impregnated with 45 mg fluorgestone acetate alone progestagens (Hashemi et al., 2006, Abecia et al., (Sponge) or with eCG (Sponge-eCG), subcutaneous 2012). Progestogen administration is common and has auricular implant of 2 mg norgestomet alone (Implant) been used with or without accompanying treatments or with eCG (Implant-eCG) or two intramuscular such as gonadatropins or prostaglandin analogs. -
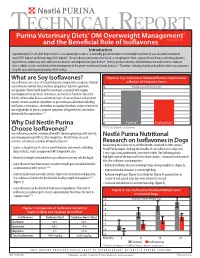
What Are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy Isoflavones Are a Class of Natural Bioactive Compounds in Soybeans
TECHNICAL REPORT Purina Veterinary Diets® OM Overweight Management® brand canine dry formula and the Beneficial Role of Isoflavones Introduction Approximately 35% of adult dogs in the U.S. are overweight or obese1. A markedly greater incidence of overweight and obesity was observed in neutered male (59% higher) and female dogs (40% higher)1. Chronic obesity can increase the risk of, or complications from, several chronic diseases including diabetes, hypertension, pulmonary and cardiovascular disease, and degenerative joint disease2. Obesity produces chronic, mild inflammation and increases oxidative stress, which, in turn, contributes to the development of the above-mentioned chronic diseases.3,4 Therefore, reducing obesity and oxidative stress can promote a long life span and improved quality of life in dogs. What are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy isoflavones are a class of natural bioactive compounds in soybeans. Natural a Marker of Oxidative Stress soy isoflavones include three chemical compounds: daidzein, genistein, 5 Plasma Isoprostanes (ng/ml) and glycitein. Many health benefits have been associated with regular consumption of soy products. In humans, soy has been found to reduce the 4 risk of cardiovascular disease and certain types of cancer (breast and prostate cancer); relieve a number of problems in post menopausal women including 3 hot flashes, osteoporosis, and decline in cognitive function; reduce cholesterol and triglycerides in plasma; improve symptoms of hypertension; and reduce 2 abdominal fat accumulation.5,6,7 1 Why Did Nestlé Purina 0 Control Isoflavones* Choose Isoflavones? *P<0.01 for Control vs. Isoflavones Soy isoflavones provide a number of benefits for managing dogs with obesity, and reducing rebound effects after weight loss. -

Applications of in Silico Methods to Analyze the Toxicity and Estrogen T Receptor-Mediated Properties of Plant-Derived Phytochemicals ∗ K
Food and Chemical Toxicology 125 (2019) 361–369 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Applications of in silico methods to analyze the toxicity and estrogen T receptor-mediated properties of plant-derived phytochemicals ∗ K. Kranthi Kumara, P. Yugandharb, B. Uma Devia, T. Siva Kumara, N. Savithrammab, P. Neerajaa, a Department of Zoology, Sri Venkateswara University, Tirupati, 517502, India b Department of Botany, Sri Venkateswara University, Tirupati, 517502, India ARTICLE INFO ABSTRACT Keywords: A myriad of phytochemicals may have potential to lead toxicity and endocrine disruption effects by interfering Phytochemicals with nuclear hormone receptors. In this examination, the toxicity and estrogen receptor−binding abilities of a QSAR modeling set of 2826 phytochemicals were evaluated. The endpoints mutagenicity, carcinogenicity (both CAESAR and ISS Toxicity models), developmental toxicity, skin sensitization and estrogen receptor relative binding affinity (ER_RBA) Nuclear hormone receptor binding were studied using the VEGA QSAR modeling package. Alongside the predictions, models were providing pos- Self−Organizing maps sible information for applicability domains and most similar compounds as similarity sets from their training Clustering and classification schemes sets. This information was subjected to perform the clustering and classification of chemicals using Self−Organizing Maps. The identified clusters and their respective indicators were considered as potential hotspot structures for the specified data set analysis. Molecular screening interpretations of models wereex- hibited accurate predictions. Moreover, the indication sets were defined significant clusters and cluster in- dicators with probable prediction labels (precision). Accordingly, developed QSAR models showed good pre- dictive abilities and robustness, which observed from applicability domains, representation spaces, clustering and classification schemes. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

List of Drugs
LIST OF DRUGS NOTE: A natural athlete DOES NOT consume any of these substances in any form at any point of his/her life. Anabolic Steroids • Anadrol • Anavar • Anderone • Andropen • 1-Androstendiol • 1-Androstendione • 4-Androstendiol • 4-Androstendione • 5-Androstendiol • 5-Androstendione • Bolazine • Bolandiol o (19-Norandrostendiol) • Bolasterone • Boldebal – Equipose • Boldenone • Boldione • Calusterone • Cardarine (PPAR) • Chioroxomesterone o (dyhdrochlormethyltesterone) • Clenbuterol o (Anabolic Agent) • Clostebol • Danazol • Deca Durabolin • Dehydrochlormethyltestosterone • Desoxymethyltestosterone • Dianabol • Dymethazine/azine (DMZ) • 5a-Dihydrostestosterone • Drostanolone • Epitestosterone o (masking agent) • Equipose • Ethisterone • Ethylestrenol • Fluoxymesterone • Formebolone • Formestane o (anti-estrogen) • Furazabol • Halodrol • 4-Hydroxy-testosterone • Mebolazine • Mestanolone • Mesterolone • Methandienone • Methandriol • Methyltestosterone • Oxandrolone • Primobolin • Stanozolol • Sustanon 250 • Winstrol • Testosterone and related compounds o Testosterone Cypionate o Testosterone Enanthate Testosterone **In any form, including injections, patches, or gels, for any reason, even if physician prescribed, No Exceptions! Growth Hormones • Pharmaceutical HGH, HCG and any other related compounds, including Insulin-Like Growth Factor 1 (IGF-1) • Oral, spray or sublingual GH compounds of pharmaceutical origin (recombinant DNA technology) Pro-Hormones, Precursors and Metabolites, Derivatives, and Related Compounds • 5-Etioallocholen-3b,7b,17b-triol. -

Summary of Product Characteristics 1. Name Of
Revised: July 2020 AN: 00391/2020 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Chronogest CR, 20 mg controlled release vaginal sponge for sheep. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each polyester polyurethane sponge contains Active substance(s) Flugestone acetate, 20 mg. List of excipients Excipients qsp 1 sponge. For a full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM Vaginal sponge. White cylindrical polyester polyurethane foam equipped with string. 4. CLINICAL PARTICULARS 4.1 Target species Sheep (ewes and ewe-lambs). 4.2 Indications for use In ewes and ewe lambs, in combination with PMSG (Pregnant Mare Serum Gonadotrophin) - Induction and synchronization of oestrus and ovulation (non cycling ewes during seasonal anoestrus and ewe lambs). - Synchronization of oestrus and ovulation (cycling ewes and ewe-lambs). 4.3 Contraindications Please refer to section 4.7 and section 4.8. 4.4 Special warnings None. Page 1 of 5 Revised: July 2020 AN: 00391/2020 4.5 Special precautions for use (i) Special precautions for use in animals - The repeated treatment with the product combined with PMSG may trigger the appearance of PMSG antibodies in some ewes. This in turn may affect the time of ovulation and result in reduced fertility when combined with fixed time artificial insemination at 55h following sponge removal. - The repeated use of sponges within one year has not been studied. - The use of a vaginal applicator designed for ewes or ewe lambs is recommended to correctly insert sponges and to avoid vaginal injuries. (ii) Special precautions to be taken by the person administering the medicinal product to animals - Direct contact with the skin should be avoided.