Development of Subtype-Selective Oestrogen Receptor-Based Therapeutics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
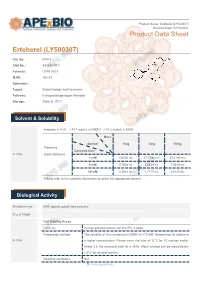
Erteberel (LY500307) Product Data Sheet
Product Name: Erteberel (LY500307) Revision Date: 01/10/2021 Product Data Sheet Erteberel (LY500307) Cat. No.: B1518 CAS No.: 533884-09-2 Formula: C18H18O3 M.Wt: 282.33 Synonyms: Target: Endocrinology and Hormones Pathway: Estrogen/progestogen Receptor Storage: Store at -20°C Solvent & Solubility insoluble in H2O; ≥14.1 mg/mL in DMSO; ≥48.3 mg/mL in EtOH Mass Solvent 1mg 5mg 10mg Preparing Concentration In Vitro Stock Solutions 1 mM 3.5420 mL 17.7098 mL 35.4195 mL 5 mM 0.7084 mL 3.5420 mL 7.0839 mL 10 mM 0.3542 mL 1.7710 mL 3.5420 mL Please refer to the solubility information to select the appropriate solvent. Biological Activity Shortsummary ERβ agonist, potent and selective IC₅₀ & Target Cell Viability Assay Cell Line: Human prostate cancer cell line (PC-3 cells) Preparation method: The solubility of this compound in DMSO is >10 mM. General tips for obtaining In Vitro a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. Reacting conditions: N/A 1 | www.apexbt.com Applications: Erteberel showed potent and selective binding affinity for ERβ with EC50 value of 0.66 nM [1]. Animal experiment Animal models: Male and female rat fertility and rat and rabbit embryo-fetal development model Dosage form: 0.03 to 10 mg/kg/day for rats, or 1 to 25 mg/kg/day for rabbits, oral gavage, for 2 or 10 weeks Applications: There were no-observed adverse effect levels following LY500307 In Vivo administration of 1 mg/kg/day for male rat fertility, 0.3 mg/kg/day for female rat fertility and embryo-fetal development, and 25 mg/kg/day for rabbit embryo-fetal development [2]. -

Targeting Fibrosis in the Duchenne Muscular Dystrophy Mice Model: an Uphill Battle
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427485; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Targeting fibrosis in the Duchenne Muscular Dystrophy mice model: an uphill battle 2 Marine Theret1#, Marcela Low1#, Lucas Rempel1, Fang Fang Li1, Lin Wei Tung1, Osvaldo 3 Contreras3,4, Chih-Kai Chang1, Andrew Wu1, Hesham Soliman1,2, Fabio M.V. Rossi1 4 1School of Biomedical Engineering and the Biomedical Research Centre, Department of Medical 5 Genetics, 2222 Health Sciences Mall, Vancouver, BC, V6T 1Z3, Canada 6 2Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Minia 7 University, Minia, Egypt 8 3Developmental and Stem Cell Biology Division, Victor Chang Cardiac Research Institute, 9 Darlinghurst, NSW, 2010, Australia 10 4Departamento de Biología Celular y Molecular and Center for Aging and Regeneration (CARE- 11 ChileUC), Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, 8331150 12 Santiago, Chile 13 # Denotes Co-first authorship 14 15 Keywords: drug screening, fibro/adipogenic progenitors, fibrosis, repair, skeletal muscle. 16 Correspondence to: 17 Marine Theret 18 School of Biomedical Engineering and the Biomedical Research Centre 19 University of British Columbia 20 2222 Health Sciences Mall, Vancouver, British Columbia 21 Tel: +1(604) 822 0441 fax: +1(604) 822 7815 22 Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427485; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

WHO Drug Information Vol
WHO Drug Information Vol. 24, No. 4, 2010 World Health Organization WHO Drug Information Contents WHO Prequalification Sitaxentan: worldwide withdrawal 307 Programmes Sibutramine: suspension of sales 307 Sibutramine-containing medicines: WHO Prequalification of Medicines withdrawal 308 Programme: survey of service Testosterone transdermal patch: quality provided to manufacturers 293 withdrawal of extension of WHO initiates pilot prequalification of indication application 308 active pharmaceutical ingredients 297 Aliskiren/valsartan: withdrawal of New on-line database for WHO marketing authorization application 308 prequalified vaccines 298 Mometasone furoate/formoterol fumarate: withdrawal of marketing Safety and Efficacy Issues authorization application 309 EMA and US FDA extend confidentiality H1N1 influenza vaccine: narcolepsy 299 arrangements indefinitely 309 Statins: interstitial lung disease 299 Tocilizumab: risk of fatal anaphylaxis 300 Recent Publications, Pioglitazone: potential bladder cancer 301 Information and Events Angiotensin receptor blockers and US Government to share patents with cancer: safety review 301 Medicines Patent Pool 310 GnRH agonists, diabetes and cardio- Clinical trials and global medicines vascular disease 301 development 310 Gadolinium-based contrast agents: Evaluation of future nanomedicines 311 kidney dysfunction 302 Reporting on opioid inaccessibility 311 Lamotrigine: aseptic meningitis Tinzaparin sodium: renal Impairment in elderly 303 Consultation Documents Tamoxifen: drug interactions involving The -

TE INI (19 ) United States (12 ) Patent Application Publication ( 10) Pub
US 20200187851A1TE INI (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No .: US 2020/0187851 A1 Offenbacher et al. (43 ) Pub . Date : Jun . 18 , 2020 ( 54 ) PERIODONTAL DISEASE STRATIFICATION (52 ) U.S. CI. AND USES THEREOF CPC A61B 5/4552 (2013.01 ) ; G16H 20/10 ( 71) Applicant: The University of North Carolina at ( 2018.01) ; A61B 5/7275 ( 2013.01) ; A61B Chapel Hill , Chapel Hill , NC (US ) 5/7264 ( 2013.01 ) ( 72 ) Inventors: Steven Offenbacher, Chapel Hill , NC (US ) ; Thiago Morelli , Durham , NC ( 57 ) ABSTRACT (US ) ; Kevin Lee Moss, Graham , NC ( US ) ; James Douglas Beck , Chapel Described herein are methods of classifying periodontal Hill , NC (US ) patients and individual teeth . For example , disclosed is a method of diagnosing periodontal disease and / or risk of ( 21) Appl. No .: 16 /713,874 tooth loss in a subject that involves classifying teeth into one of 7 classes of periodontal disease. The method can include ( 22 ) Filed : Dec. 13 , 2019 the step of performing a dental examination on a patient and Related U.S. Application Data determining a periodontal profile class ( PPC ) . The method can further include the step of determining for each tooth a ( 60 ) Provisional application No.62 / 780,675 , filed on Dec. Tooth Profile Class ( TPC ) . The PPC and TPC can be used 17 , 2018 together to generate a composite risk score for an individual, which is referred to herein as the Index of Periodontal Risk Publication Classification ( IPR ) . In some embodiments , each stage of the disclosed (51 ) Int. Cl. PPC system is characterized by unique single nucleotide A61B 5/00 ( 2006.01 ) polymorphisms (SNPs ) associated with unique pathways , G16H 20/10 ( 2006.01 ) identifying unique druggable targets for each stage . -

New Insights for Hormone Therapy in Perimenopausal Women Neuroprotection
Chapter 12 New Insights for Hormone Therapy in Perimenopausal Women Neuroprotection Manuela Cristina Russu and Alexandra Cristina Antonescu Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.74332 Abstract Perimenopause is a mandatory period in women’s life, when the medical staff may initiate hormone therapy with sex steroids for the delay of brain aging and neurodegenerative diseases, during the so-called “window of opportunity.” Animals’ models are helpful to sustain the still controversial results of human clinical observational and/or randomized controlled studies. Estrogens, progesterone, and androgens, with their nuclear and mem- brane receptors, genes, and epigenetics, with their connections to cholinergic, GABAergic, serotoninergic, and glutamatergic systems are involved in women’snormalbrainorin brain’s pathology. The sex steroids are active through direct and/or indirect mechanisms to modulate and/or to protect brain plasticity, and vessels network, fuel metabolism—glucose, ketones, ATP, to reduce insulin resistance, and inflammation of the aging brain through blood-brain barrier disruption, microglial aberrant activation, and neural cell survival/loss. Keywords: perimenopause, “window” of opportunity, neuroprotection, sex steroid hormones 1. Introduction The months/years of perimenopause represent an important moment during women’saging, when sex steroids and their receptors decline are evident in the hippocampal and cortical neu- rons, after estrogen exposure during the reproductive years. The sex steroid hormones decline is associated/acts synergic to other factors as hypertension, diabetes, hypoxia/obstructive sleep apnea, obesity, vitamin B12/folate deficiency, depression, and traumatic brain injury to promote diverse pathological mechanisms involved in brain aging, memory impairment, and AD. -

Estrogen Receptor Beta Is a Negative Regulator of Mammary Cell Proliferation Xiaozheng Song University of Vermont
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2014 Estrogen Receptor Beta Is A Negative Regulator Of Mammary Cell Proliferation Xiaozheng Song University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Animal Sciences Commons, and the Molecular Biology Commons Recommended Citation Song, Xiaozheng, "Estrogen Receptor Beta Is A Negative Regulator Of Mammary Cell Proliferation" (2014). Graduate College Dissertations and Theses. 259. https://scholarworks.uvm.edu/graddis/259 This Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. ESTROGEN RECEPTOR BETA IS A NEGATIVE REGULATOR OF MAMMARY CELL PROLIFERATION A Dissertation Presented by Xiaozheng Song to The Faculty of the Graduate College of The University of Vermont In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Specializing in Animal Science October, 2014 Accepted by the Faculty of the Graduate College, The University of Vermont, in partial fulfillment of the requirements for the degree of Doctor of Philosophy, specializing in Animal Science. Dissertation Examination Committee: ____________________________________ Advisor Zhongzong Pan Ph.D. ____________________________________ Co-Advisor Andre-Denis Wright Ph.D. ____________________________________ Rona Delay, Ph.D. ____________________________________ David Kerr, Ph.D. ____________________________________ Chairperson Karen M. Lounsbury, Ph. D. ____________________________________ Dean, Graduate College Cynthia J. Forehand, Ph.D. Date: May 5, 2014 ABSTRACT The mammary gland cell growth and differentiation are under the control of both systemic hormones and locally produced growth factors. -

Estriol Therapy for Autoimmune and Neurodegenerative Diseases and Disorders
(19) TZZ¥Z__T (11) EP 3 045 177 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 20.07.2016 Bulletin 2016/29 A61K 31/565 (2006.01) A61K 31/566 (2006.01) A61K 31/568 (2006.01) A61K 45/06 (2006.01) (2006.01) (2006.01) (21) Application number: 16000349.7 A61K 31/785 A61P 25/00 (22) Date of filing: 26.09.2006 (84) Designated Contracting States: (72) Inventor: Voskuhl, Rhonda R. AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Los Angeles, CA 90024 (US) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR (74) Representative: Müller-Boré & Partner Patentanwälte PartG mbB (30) Priority: 26.09.2005 US 720972 P Friedenheimer Brücke 21 26.07.2006 US 833527 P 80639 München (DE) (62) Document number(s) of the earlier application(s) in Remarks: accordance with Art. 76 EPC: This application was filed on 11-02-2016 as a 13005320.0 / 2 698 167 divisional application to the application mentioned 06815626.4 / 1 929 291 under INID code 62. (71) Applicant: The Regents of the University of California Oakland, CA 94607 (US) (54) ESTRIOL THERAPY FOR AUTOIMMUNE AND NEURODEGENERATIVE DISEASES AND DISORDERS (57) The present invention relates to a dosage from comprising estriol and glatiramer acetate polymer-1 for use in the treatment of multiple sclerosis (MS), as well as to a kit comprising estriol and glatiramer acetate polymer-1. EP 3 045 177 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 045 177 A1 Description [0001] This invention was made with Government support under Grant No. -

Downloaded from Survive Nursing | Survivenursing.Com V20110426
Generic Stem Stem Definition Examples -abine (see -arabine, -citabine) decitabine -ac Anti-inflammatory agents (acetic acid derivatives) bromfenac; dexpemedolac -acetam See -racetam -actide Synthetic corticotropins seractide -adol or -aldol- Analgesics (mixed opiate receptor agonists/ antagonists) tazadolene; spiradolene; levonantradol -adox Antibacterials (quinoline dioxide derivatives) carbadox -afenone Antiarrhythmics (propafenone derivatives) alprafenone; diprafenone -afil PDE5 inhibitors tadalafil -aj- Antiarrhythmics (ajmaline derivatives) lorajmine -aldrate Antacid aluminum salts magaldrate -algron Alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol Combined alpha and beta blockers labetalol; medroxalol -amivir (see -vir) -ampa Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) -ampanel Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; becampanel antagonists -ampator Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; forampator modulators -andr- Androgens nandrolone -anib Angiogenesis inhibitors semaxanib -anserin Serotonin 5-HT2 receptor antagonists altanserin; tropanserin; adatanserin -antel Anthelmintics (undefined group) carbantel -antrone Antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine Antineoplastics (arabinofuranosyl derivatives) fazarabine; fludarabine aril-, -aril, -aril- Antiviral (arildone derivatives) pleconaril; arildone; fosarilate -arit Antirheumatics (lobenzarit type) lobenzarit; clobuzarit -arol -

Datasheet Inhibitors / Agonists / Screening Libraries a DRUG SCREENING EXPERT
Datasheet Inhibitors / Agonists / Screening Libraries A DRUG SCREENING EXPERT Product Name : Prinaberel Catalog Number : TQ0149 CAS Number : 524684-52-4 Molecular Formula : C15H10FNO3 Molecular Weight : 271.24 Description: Prinaberel (ERB-041) is an effective and selective ERβ agonist and >200-fold selective for ERβ. Storage: 2 years -80°C in solvent; 3 years -20°C powder; Solubility DMSO ≥38mg/mL(140.1 mM) ( < 1 mg/ml refers to the product slightly soluble or insoluble ) In vitro Activity Treatment with ERβ selective estrogen agonists liquiritigenin and ERB-041 reduced the ability to invade a reconstituted basement membrane and to migrate in response to the cellular stimulus [1]. Pretreatment the PMs with ERB-041 resulted in significant inhibition of LPS-induced iNOS expression and NF-kappaB activation by preventing its nuclear translocation [3]. In vivo Activity Tumor numbers and volume were reduced by 60% and 84%, respectively, in the Erb-041-treated group as compared with UVB (alone) control. This inhibition in tumorigenesis was accompanied by a decrease in proliferating cell nuclear antigen (PCNA), cyclin D1, VEGF, and CD31, and an increase in apoptosis [2]. Reference 1. Hinsche O, et al. Estrogen receptor β selective agonists reduce invasiveness of triple-negative breast cancer cells. Int J Oncol. 2015 Feb;46(2):878-84. 2. Chaudhary SC, et al. Erb-041, an estrogen receptor-β agonist, inhibits skin photocarcinogenesis in SKH-1 hairless mice by downregulating the WNT signaling pathway. Cancer Prev Res (Phila). 2014 Feb;7(2):186-98. 3. Xiu-li W, et al. ERB-041, a selective ER beta agonist, inhibits iNOS production in LPS-activated peritoneal macrophages of endometriosis via suppression of NF-kappaB activation. -

Decreases Breast Cancer Cell Survival by Regulating the IRE1&Sol
Oncogene (2015) 34, 4130–4141 © 2015 Macmillan Publishers Limited All rights reserved 0950-9232/15 www.nature.com/onc ORIGINAL ARTICLE ERβ decreases breast cancer cell survival by regulating the IRE1/XBP-1 pathway G Rajapaksa1, F Nikolos1, I Bado1, R Clarke2, J-Å Gustafsson1 and C Thomas1 Unfolded protein response (UPR) is an adaptive reaction that allows cancer cells to survive endoplasmic reticulum (EnR) stress that is often induced in the tumor microenvironment because of inadequate vascularization. Previous studies report an association between activation of the UPR and reduced sensitivity to antiestrogens and chemotherapeutics in estrogen receptor α (ERα)- positive and triple-negative breast cancers, respectively. ERα has been shown to regulate the expression of a key mediator of the EnR stress response, the X-box-binding protein-1 (XBP-1). Although network prediction models have associated ERβ with the EnR stress response, its role as regulator of the UPR has not been experimentally tested. Here, upregulation of wild-type ERβ (ERβ1) or treatment with ERβ agonists enhanced apoptosis in breast cancer cells in the presence of pharmacological inducers of EnR stress. Targeting the BCL-2 to the EnR of the ERβ1-expressing cells prevented the apoptosis induced by EnR stress but not by non-EnR stress apoptotic stimuli indicating that ERβ1 promotes EnR stress-regulated apoptosis. Downregulation of inositol-requiring kinase 1α (IRE1α) and decreased splicing of XBP-1 were associated with the decreased survival of the EnR-stressed ERβ1-expressing cells. ERβ1 was found to repress the IRE1 pathway of the UPR by inducing degradation of IRE1α.