2015 Field Day Proceedings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proceedings NCAFST
UGC sponsored and DBT funded National Conference on Advances in Food Science and Technology (NCAFST’2016) 16-17 March, 2016 Edited and Compiled by : Ms. Para Dholakia Ms. Saumya Chaturvedi Ms. Chaynika Verma Ms. Vandana Department of Food Technology Shaheed Rajguru College of Applied Sciences for Women University of Delhi Printed & Published by : Cherub Digimax B-1191, G. D. Colony Mayur Vihar Phase-3 Delhi - 110096 © Shaheed Rajguru College of Applied Sciences for Women, Delhi Edition : 2016 Every possilbe effort has been made to ensure that the information in this book is accurate. The publisher and author cannot accept responsibility for any errors or omissions however caused. No responsibility for loss/damage caused to any person as a result of the material in this publication can be accepted by the editor, publisher or the author. SRCASW, Delhi Foreword by Patron It is a matter of great pride and honour that Department of Food Technology, Shaheed Rajguru College of Applied Sciences for Women is hosting the UGC and DBT sponsored “National Conference on Advances in Food Science and Technology” on 16th and 17th March, 2016. The aim of this conference is to develop and motivate students towards research and innovation and provide a platform for faculty members of various institutions to exchange their expertise and ideas. This conference is a high profile event attracting students of undergraduate and postgraduate level, research scholars, academicians and industry professionals from all over the country. Such conferences play an important role in apprising the students of the latest developments in their discipline. The papers contributed for the conference are from the vast field of Food Science and Technology including emerging food processing technologies, food safety, nutrition, functional foods, gluten free bakery products, novel product development, designer foods and food packaging. -

Ema Parties & Events 10.17
PARTIES & SPECIAL EVENTS 74 W ILLINOIS ST, CHICAGO, IL (312) 527-5586 EMACHICAGO.COM WELCOME Ema is a Mediterranean restaurant showcasing Chef CJ Jacobson’s lighter California style of cooking. At the core of the menu are spreads, dips and mezze, Mediterranean small plates. Signature dishes include Spicy Hummus, House-Made Stracciatella, Grilled Octopus, and Kefta Kebabs. The open and inviting dining room features reclaimed wood, rustic white brick and an indoor ivy terrace with operable windows. The dining room accommodates groups up to 150 guests and may be custom configured for your next family-style dinner, business lunch or cocktail reception event. CONTACTS KRISTA ROMINGER sales and catering manager 312.254.6486 [email protected] ELSA JEROUSEK sales and catering manager 312.485.5778 [email protected] 74 W ILLINOIS ST, CHICAGO, IL • (312) 527-5586 • EMACHICAGO.COM FLOOR MAP OUTDOOR SEATING OUTDOOR SEATING AREA SEATED RECEPTION AREA SEATED RECEPTION A 16 20 D 25 35 B 52 75 E X 40 C 58 100 F 22 35 74 W ILLINOIS ST, CHICAGO, IL • (312) 527-5586 • EMACHICAGO.COM PASSED APPETIZERS MEZZE priced by the dozen GREEN FALAFEL avocado tzatziki, garlic tahini, dhania spice - $30 CRISPY POTATOES mizithra, rosemary, scallion crema - $28 HOUSE-MADE STRACCIATELLA mighty vine tomatoes, croutons, shallots, sherry vinegar, basil - $32 PAN-ROASTED ROMANESQUE CAULIFLOWER house yogurt, local honey - $30 SEAFOOD priced by the dozen SEASONAL CRUDO ask about our seasonal offerings - $38 GRILLED OCTOPUS fried kale, fingerlings, preserved lemon vinaigrette -

Remote Desktop Redirected Printer
Merlino Foods 1 Summer Product Book Page: Appetizer Appetizer Breaded Cut Okra OKRABREADED Westpac 12/24 OZ Kronos Spanokopita (1oz Portions) SPANO Kronos 144/1 OZ L&W Empty Steam Bun Folded STEAMBUN L & W 22/12 CT Packer S/O Pork Gyoza GYOZA Packer 4/1.98 LB Packer Dolmades Stuffed Vine Grape Leaves (60 Per Can) DOLMADE Packer 6/70 OZ Stonefire S/O Naan Dippers Bite Size NAANBITE Stonefire 340/.35 OZ Supreme Vegetarian Spring Roll VEGSPRINGROLL Supreme 1/200 CT Artichokes Maria/Ambrosia Artichoke Bottoms (7-9 Ct Per Can) ARTBOT Maria 24/14 OZ Merlino Artichoke Hearts (50-60 Ct Per Can) ART5060 Merlino 6/3 KG Merlino Artichoke Hearts Quartered ARTQUART Merlino 6/3 KG Merlino Marinated Artichoke Hearts Quartered ARTMAR610 Merlino 6/3 KG Orto Baby Artichokes With Stems (Imported) ARTBABY Orto 6/5.5 LB Roland Quartered Artichoke Hearts (Retail) ARTQUART1214 Roland 12/14 OZ Asian Cookies/Crackers Golden S/O Fortune Cookies Portion Control FORTUNECOOKIE Golden 1/7 LB Hot S/O Kid Nori Flavor Rice Crackers RICECRACKERNORI Hot Kid 20/5.64 OZ Flours/Starches Blue Star Mochiko Rice Flour RICEFLOUR36 Blue Star 36/16 OZ Golden Anchor Large Pearl Tapioca TAPIOCAPEARLLG Golden 50/14 OZ Golden Anchor Small Pearl Tapioca TAPIOCAPEARL Golden 50/14 OZ Golden Anchor Tapioca Starch (Flour) TAPIOCAFLOURP Golden 50/14 OZ Misc. Custom Sake Kasu SAKE LEES KASU Custom 1/5 LB Double S/O Happiness Brand Chinese Doughnuts DOUGHNUTCHIN Tom 17/3 CT Ginger L/S People Ginger Juice GINGERJUICE Ginger Peo 12/5 OZ Golden Palm Sugar No Syrup PALMSUGAR Golden 30/16 OZ Asian Misc. -

Vegan Lunch Menu Lunch Menu
APÉRITIF TO START GLASS OF PROSECCO (125ml) .................................6.65 GREEK FLATBREAD ...................................................... 3.25 GREEK OLIVES ................................................................ 3.00 FILOXENIA DINNER MENU 314kcal V VG GF OPA PROSECCO BELLINI (125ml) .....................................6.65 533kcal V VG FOUR DISHES FOR 13.30pp NEGRONI ........................................................................6.75 Add olive oil & Dukkah, a spicy mix of ground, CRUDITÉS ........................................................................3.25 APEROL SPRITZ ...........................................................6.90 dry roasted nuts and seeds. 621kcal V VG ....................... 3.50 Fresh-cut carrot, celery and cucumber. 47kcal V VG GF AVAILABLE SUNDAY - THURSDAY FROM 5.00PM FANTASTIC EVENING WE RECOMMEND 3 OR 4 MEZE PER PERSON. COLD MEZE ARE SERVED FIRST, HOT FOLLOWS WHEN READY. PERFECT FOR SHARING 1. CHOOSE A DISH: MEAL Greek Flatbread V VG or Crudités V VG GF DEAL COLD MEZE HOT MEZE 2. CHOOSE ONE OF THESE COLD MEZE: Santorini Fava V VG GF or Green Pea Fava V VG GF or TARAMASALATA ........................................................... 4.55 Houmous V VG GF or Tzatziki V GF or Spicy Feta Dip Our daily creamy blend with naturally undyed (Htipiti) V GF or Melitzanosalata V VG GF or Taramasalata cod roe. It’s not meant to be pink! 989kcal GIGANDES WITH SPINACH .........................................5.25 CHICKEN MONASTIRAKI .................................................. 6.90 3. CHOOSE ONE OF THESE HOT MEZE: SANTORINI FAVA .................................................... 4.75 Hearty giant beans and spinach, cooked in a tomato Chicken, marinated with Greek herbs, served with tzatziki, Chicken Skewer GF or BBQ Chicken Wings GF Yellow lentils from Santorini, cooked and blended with herbs and garlic sauce. 540kcal V VG GF onion and tomatoes. 245kcal GF or Chicken Monastiraki GF or Loukaniko Beef & and spices, topped with Santorini capers & onions. -

Group Participation Required $22 Per Person ++ First Second Third Fresh
$22 per person ++ Choose 1 item per course cocktails first bloody mary 10 all spreads are served with flatbread or crudité mimosa 9 melitzanosalata smoked eggplant, roasted peppers, walnuts, feta choice of: orange / grapefruit / favosalata yellow lentils, scallions, black garlic, pine nuts pineapple tzatziki yogurt, cucumber, dill, citrus french 75 10 hummus chickpea, tahini, sultan chutney gin, lemon, sparkling wine second kir royal 10 creme de casis, sparkling wine greek caesar baby romaine, feta, croutons frittata crispy lamb, potatoes, feta aperol sprtiz 10 omelette tomato, avocado, chickpea, kale aperol, orange, soda water, sparkling wine spano-scramble leek, spinach, feta, flatbread chip greek bagel & lox taramasalata, salmon, caper, tomato third baklava french toast walnuts, seasonal fruit fresh juice 5 ice cream and sorbet seasonal flavors orange, grapefruit, pineapple fruit plate seasonal fruit, greek yogurt group participation required *consuming raw or undercooked meats, poultry, seafood, shellfish or eggs may increase your risk of foodborne illness A 20% gratuity may be added for parties of 6 or more. 4900 hampden lane bethesda, md 20814 www.kapnoskouzina.com @kapnoskouzina $22 per person ++ option one option two choose 1 item per course choose 1 item per course first first tzatziki yogurt, cucumber, dill, citrus greek caesar baby romaine, feta, croutons hummus chickpea, tahini, sultan chutney maroulosalata mixed heirloom greens, mizithra cheese, citrus vinaigrette melitzanosalata smoky eggplant, roasted peppers, walnuts, -

Dec/Jan 2008
SPECIAL SECTION 2008 Specialty Cheese Guide Dec./Jan. ’08 Deli $14.95 BUSINESS Also Includes The American Cheese Guide ALSO INSIDE Entrées Natural Meats Italian Deli Salami Reader Service No. 107 DEC./JAN. ’08 • VOL. 12/NO. 6 Deli TABLE OF CONTENTS BUSINESS FEATURES Merchandising Entrées In The Deli ..............17 Fresh is the buzzword sparking a revolution in today’s supermarket industry. COVER STORY PROCUREMENT STRATEGIES Natural Deli Meats ........................................59 More retailers are responding to consumer concern for both a more healthful product and animal welfare. MERCHANDISING REVIEW Viva Italy! ......................................................63 Learning about the background of imported Italian deli products spurs effective marketing and increased profits. DELI MEATS Salami And Cured Meat: Renaissance With An Ethnic Flair ..................69 Effectively merchandise a range of salami and cured meats as high-end unique products. SPECIAL SECTION......................19 1122 2008 COMMENTARY EDITOR’S NOTE Specialty The Specialty Cheese Challenge/Opportunity..................................6 Cheese Guide It may sound like a burden — can’t we just sell product? — but it really is the opportunity. PUBLISHER’S INSIGHTS 2008 Will Be An Interesting Year...................8 From cause marketing and the invasion of the Brits to the greening of politics, 2008 will prove to be a pivotal year. MARKETING PERSPECTIVE There’s No Place Like You For The Holidays ..................................73 You can mount any merchandising -

Georges Main Menu Approved
Dips (each) Tempura Fried Feta tzatziki, taramasalata, hummus, with peppered honey spicy feta or tahini Premium meat cuts, aged by our Skewer Platter Gyro Platter • add pita bread White Bait (when available) butcher also available for your home handmade skewers served with shaved meat served with fresh cut deep fried fresh cut chips, pita bread, sliced tomato, chips, pita bread, sliced tomato, red Calamari red onion and a choice of dip onion and a choice of dip grilled or lightly fried George’s Stack STEAK BASTING GEORGE'S BASTING PEPPERED CRUST with homemade aioli sauce thinly sliced brinjal, Beef Fillet | Chicken | Pork Beef | Chicken | Pork speciality barbecue seasoning olive oil • oregano • herbs crushed red and black pepper crispy fried with grated Baby Squid Heads kefalograviera cheese & tzatziki lightly fried with homemade aioli sauce Beetroot Falafel served with a side of choice deep fried chickpea beetroot balls minimum 21 days matured pure beef wet-aged handcut by our master butcher Octopus ( when available ) served with tahini served with a side of choice coal fired with oregano and olive oil, served George’s Beef Burger ` with taramasalata Fish Croquettes Rump 250g 250g chargrilled premium beef, gherkins, cheddar cheese, tomato, served with tzatziki 350g red onion and special burger sauce Cheese Pie • add bacon oven baked cheese pie Mussel Saganaki Fillet 200g 300g with feta cheese, tomato and ouzo Gyro Burger Saganaki served with pita bread pan seared kefalograviera cheese Sirloin 250g beef/chicken/pork -

Champagne Trays Loukaniko 6 Your Choice of Bubbles with Fresh Juices and Garnishes 54
Breakfast Pies | Πίτες Brunch | Καλημέρα Bougatsa 6 Baklava Oatmeal 10 phyllo, custard, ground cinnamon, powdered sugar rolled oats, toasted walnuts, cinnamon-allspice, cloves, Mushroom Pie 14 Kalamata figs, honey, shredded phyllo seasonal mushrooms, green garlic, phyllo Koulouri and Lox 12 Tyropita 10 manouri cream cheese, scallions, tomatoes, red onions, Greek phyllo cheese pie capers, arugula, Greek bagel Greek Yogurt Pancakes 14 For the Table | Για το Τραπέζι vyssino, honey, toasted almonds, fresh berries Greek Cheese Plate 16 Tsoureki Toast half/full 14/28 kasseri, manouri, feta, dried fruits, marmalade, honey, fresh berries, maple whipped cream, add merenda +2 ginger-cranberry toast Classic Greek Omelet 14 Tzatziki Trio 14 spinach, dill, scallions, feta, served with charred pineapple, spicy pepper and traditional tzatziki homefries and pita Mango Melitzanosalata 12 Kayianas 12 charred mango and eggplant, tomatinia scrambled eggs with tomatoes, olives and feta, Tyrokafteri 8 grated mizithra, arugula, pita spicy whipped feta, Florina peppers Shakshouka (serves 2 or more) 24 Dolmades 8 eggs baked in a pan of spiced tomato sauce, peppers, rice, sumac, pine nut, smoked yogurt onions with feta and pita Okra Horiatiki 14 Greek-Style Chicken and Biscuits 14 tomato, cucumber, red onion, Kalamata olives, feta, fried drumsticks, Greek yogurt biscuits, loukaniko gravy rigani, Greek olive oil, okra crisps Three-Eggs 14 Feta 14 your choice of loukaniko or bacon, served with sesame encrusted feta, Greek honey homefries and pita Zucchini Crisps -

Meze Platter for Two (Veg) 34 Hummus, Tzatziki, Dolma, Falafel, Tahini, Spanakopita, Feta Cheese, and Greek Olives
MEZÉ’S Add fresh vegetable slices 3 HUMMUS (GF,VEG) 8 CALAMARI 11 Garbanzo beans, sesame paste (tahini), garlic, and Lightly breaded and golden fried squid with spicy lemon juice. Served with warm pita tzatziki CILANTRO JALAPENO HUMMUS (GF, VEG) 8 LAMB CHOPS (GF) 14 Served with warm pita Grilled lamb chops f inished with latho lemono TOMATO BASIL HUMMUS (GF, VEG) 8 SOUVLAKIA (Greek Kabob) (GF) Hummus Contest winner. Served with warm pita CHICKEN 11 / FILET 13 Mini skewers of protein served with tzatziki and FIERY FETA (GF, VEG) 8 pita Feta cheese, roasted red pepper, and garlic. Served with warm pita FIERY FETA MAC’ N CHEESE (VEG) 11 Penne pasta, kasseri, cheddar, f irey feta, and pita BABA GHANOUSH (GF, VEG) 8 crumbs (add bacon 1) Grilled eggplant, tahini, garlic, and lemon juice. Served with warm pita SPANAKOPITA (VEG) 11 Puff pastry f illed with fresh spinach, leeks, TZATZIKI (GF, VEG) 8 feta cheese, mint, dill, and served with tzatziki Greek yogurt, cucumber, garlic, dill, and mint. Served with warm pita DOLMATHES (GF) 14 Grape leaves stuffed with ground sirloin, rice, mint, FETA & OLIVES (GF, VEG) 10 dill, with avgolemono sauce Rousas feta cheese and assorted Greek olives GARLIC MASHED POTATOES (GF, VEG) 7 DOLMA (GF, VEG) 11 Roasted garlic and mizithra cheese Rice, green onion, parsley, dill, garlic mixed and wrapped in grape leaves POTATOES LEMONATO (GF) 7 Fried potatoes tossed in lemon juice and fresh herbs FALAFEL (GF, VEG) 9 Chickpea croquet, served with tahini and pita MEZE FRIES (VEG) 9 Hand cut and tossed in garlic, fresh parsley, topped SAGANAKI (VEG) 16 with feta cheese Pan seared kasseri cheese, flambéed in Metaxa brandy GREEK QUESADILLA (VEG) 9 Pita bread, feta, kasseri, cheddar, bell peppers, SHRIMP & OUZO SAGANAKI (GF) 19 onion, and tomato. -
Cultures Reference Chart (Lactic Cultures)
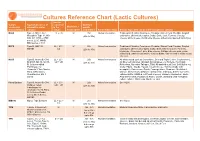
Cultures Reference Chart (Lactic Cultures) Species Culture Equivalent names of contained Optimum stocked by alternative starter in the Mesophile / growth Cheesemaking cultures culture Thermophile temperature Fermentation Type Cheese types that can be made using these cultures M265 Type A, MA11, C61, LL + LC M 25c Homofermentative Traditional Cheddar, Farmhouse Cheddar, Stirred Curd Cheddar, English Mesophile Type 11, M0 (20c to 35c) acid styles, UK territorial styles, Colby, Brick, Jack, Farmers, Cottage 030, R7, R703 – R708 cheese, white cheese, Continental cheese, milled curd, washed curd styles series, LL50, MWO, MA0 series, C 101 M272 Type B, MM 100, LL + LC + M 25c Heterofermentative Traditional Cheddar, Farmhouse Cheddar, Stirred Curd Cheddar, English MM101 LD (20c to 35c) acid styles, UK territorial styles, Colby, Brick, Monterey Jack, Farmers, Limburger, Camembert, Brie, Blue cheese, Cottage cheese, sour cream, milled curd, washed curd styles, cultured butter, can be used in white mould cheeses, M235 Type B, Aroma B, C64, LL + LC + M 25c Heterofermentative All white mould such as Camembert, Brie and Triple Cream, Coulommiers, M O30R, M0 36, M0 36 LD + LM (20c to 35c) All Blue vein varieties, All wash rind varieties e.g. Raclette, Port Salut, R, Cultures called Reblochon, Muenster Taleggio, Tilsit, All washed curd Semi Soft Styles e.g. 'Farmhouse' or Colby, Edam., Gouda, Havarti, Cream cheese, Tomme (made with 'Aromatic', Flora Danica, mesophile), Sour cream, Quark, Fromage blanc, Chaource, Neufchatel, Flora, CHN Series, Chevre, -

Cretan Dinner
CRETAN DINNER STARTERS SALADS Chicken Soup Spinach 10,00€ 14,00€ With coarsely cooked tomatoes in the wood Hearts of spinach arugula, pomegranate, oven and pure virgin olive oil. crushed sour mizithra, almond, jelly from strawberry and orange fillets with pumpkin seeds. Flute 10,00€ Potato salad With greens and fennel on cream cheesse. 15,00€ Salad of broken boiled potatoes, summer toma- Liver toes, cucumber, onion and egg with 14,00€ pure virgin olive oil and village vinegar. Savory liver with caramelized onions & raisins. Snails 11,00€ PASTA With coarse salt, rosemary and vinegar. Skioufichta Haniotiko mpoureki 14,00€ 12,00€ Cretan traditional pasta cooked with tomato, Zucchini and Potato pie from Chania. served with cream cheese. Zucchini blossoms Magiri 13,00€ 16,00€ Stuffed with wild artichokes vegetables, rice and Round shapped Cretan pasta, with rabbit, light yoghurt mousse. and dry cream cheese. Meatballs Pilaf 12,00€ 14,00€ Minced beef with sour & mint served with With smoked apaki lime and saffron. smoked paprika emulsion. Cretan Tacos 12,00€ With lightly cooked lamb and potato. MAIN COURSES DESSERTS Organic Chicken Baklava 18,00€ 11,00€ Stuffed with Cretan gruyere, sun-dried tomatoes With walnuts and fresh vanilla ice cream with and olives on spinach & cream. cinnamon. Beef Sfakiani pie 19,00€ 11,00€ Roasted in dessert wine and round maggiri Traditional pie from Sfakia, stuffed with sweet with gruyere & okra. mizithra served with honey. Lamb shank Cool pie 22,00€ 11,00€ Simmered in the wood oven with herbs and With sheep yoghurt and sour cherries. papardelles cooked in its own juice with tomato and Cretan cream cheese. -
Top 10 Crete
EYEWITNESS TRAVEL TOP10 CRETE N O ORO ID S U K B O OF M OR I EN 10 5 A UT LIKO MA 2 MA LI Best beaches K Agios E O S OUT PLATIA I Titos AGIOS I TOU ARI ADNI AS TITOS S T S 10 R IO IGI O Must-see museums & ancient sites AY F M I R A B E L O U Battle of Crete O B Loggia AN Museum S 10 O Venetian DHR K Spectacular areas of natural beauty HA M D ZID A K I U Walls IL DOU OG ATO O U S D EO HÍ D 10 K Best traditional tavernas D O Archaeological EDHALOU RA I APOUTIE Museum S THOU IDOMENEO N A 10 D Most exciting festivals 10 Liveliest bars & clubs 10 Best hotels for every budget 10 Most charming villages 10 Fascinating monasteries & churches 10 Insider tips for every visitor YOUR GUIDE TO 10THE 10 BEST OF EVERYTHING TOP 10 CRETE ROBIN GAULDIE EYEWITNESS TRAVEL Left Dolphin fresco, Knosos Right Rethymno harbour Contents Crete’s Top 10 Contents Ancient Knosos 8 Irakleio 12 Produced by Blue Island Publishing Reproduced by Colourscan, Singapore Printed Irakleio Archaeological and bound in China by Leo Paper Products Ltd First American Edition, 2003 Museum 14 11 12 13 14 10 9 8 7 6 5 4 3 2 1 Chania 18 Published in the United States by DK Publishing, 375 Hudson Street, Phaestos 20 New York, New York 10014 Reprinted with revisions Rethymno 22 2005, 2007, 2009, 2011 Gortys 24 Copyright 2003, 2011 © Dorling Kindersley Limited Samaria Gorge 26 All rights reserved.