Management of Cardiac Toxicity Induced by Chemotherapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Chronic Myelogenous Leukemia in Pregnancy
ANTICANCER RESEARCH 35: 1-12 (2015) Review Management of Chronic Myelogenous Leukemia in Pregnancy AMIT BHANDARI, KATRINA ROLEN and BINAY KUMAR SHAH Cancer Center and Blood Institute, St. Joseph Regional Medical Center, Lewiston, ID, U.S.A. Abstract. Discovery of tyrosine kinase inhibitors has led to Leukemia in pregnancy is a rare condition, with an annual improvement in survival of chronic myelogenous leukemia incidence of 1-2/100,000 pregnancies (8). Since the first (CML) patients. Many young CML patients encounter administration of imatinib (the first of the TKIs) to patients pregnancy during their lifetime. Tyrosine kinase inhibitors with CML in June 1998, it is estimated that there have now inhibit several proteins that are known to have important been 250,000 patient years of exposure to the drug (mostly functions in gonadal development, implantation and fetal in patients with CML) (9). TKIs not only target BCR-ABL development, thus increasing the risk of embryo toxicities. tyrosine kinase but also c-kit, platelet derived growth factors Studies have shown imatinib to be embryotoxic in animals with receptors α and β (PDGFR-α/β), ARG and c-FMS (10). varying effects in fertility. Since pregnancy is rare in CML, Several of these proteins are known to have functions that there are no randomized controlled trials to address the may be important in gonadal development, implantation and optimal management of this condition. However, there are fetal development (11-15). Despite this fact, there is still only several case reports and case series on CML in pregnancy. At limited information on the effects of imatinib on fertility the present time, there is no consensus on how to manage and/or pregnancy. -

Nilotinib (Tasigna®) EOCCO POLICY
nilotinib (Tasigna®) EOCCO POLICY Policy Type: PA/SP Pharmacy Coverage Policy: EOCCO136 Description Nilotinib (Tasigna) is a Bcr-Abl kinase inhibitor that binds to, and stabilizes, the inactive conformation of the kinase domain of the Abl protein. Length of Authorization Initial: Three months Renewal: 12 months Quantity Limits Product Name Dosage Form Indication Quantity Limit Newly diagnosed OR resistant/ intolerant 50 mg capsules 112 capsules/28 days Ph+ CML in chronic phase nilotinib 150 mg capsules Newly diagnosed Ph+ CML in chronic phase 112 capsules/28 days (Tasigna) Resistant or intolerant Ph + CML 200 mg capsules 112 capsules/28 days Gastrointestinal Stromal Tumors (GIST) Initial Evaluation I. Nilotinib (Tasigna) may be considered medically necessary when the following criteria are met: A. Medication is prescribed by, or in consultation with, an oncologist; AND B. Medication will not be used in combination with other oncologic medications (i.e., will be used as monotherapy); AND C. A diagnosis of one of the following: 1. Chronic myelogenous leukemia (CML) ; AND i. Member is newly diagnosed with Philadelphia chromosome-positive (Ph+) or BCR-ABL1 mutation positive CML in chronic phase; OR ii. Member is diagnosed with chronic OR accelerated phase Ph+ or BCR-ABL1 mutation positive CML; AND a. Member is 18 years of age or older; AND b. Treatment with a tyrosine kinase inhibitor [e.g. imatinib (Gleevec)] has been ineffective, contraindicated, or not tolerated; OR iii. Member is diagnosed with chronic phase Ph+ or BCR-ABL1 mutation positive CML; AND a. Member is one year of age or older; AND 1 nilotinib (Tasigna®) EOCCO POLICY b. -

And Cisplatin-Resistant Ovarian Cancer
Vol. 2, 607–610, July 2003 Molecular Cancer Therapeutics 607 Alchemix: A Novel Alkylating Anthraquinone with Potent Activity against Anthracycline- and Cisplatin-resistant Ovarian Cancer Klaus Pors, Zennia Paniwnyk, mediated stabilization of the topo II-DNA-cleavable complex Paul Teesdale-Spittle,1 Jane A. Plumb, and resulting in inhibition of poststrand passage DNA religa- Elaine Willmore, Caroline A. Austin, and tion (1). This event is not lethal per se but initiates a cascade 2 Laurence H. Patterson of events leading to cell death (2). Anthraquinones, as ex- Department of Pharmaceutical and Biological Chemistry, The School of emplified by mitoxantrone, are topo II inhibitors with proven Pharmacy, University of London, London WC1N 1AX, United Kingdom [K. P., L. H. P.]; Department of Pharmacy, De Montfort University, success for the treatment of advanced breast cancer, non- Leicester LE1 9BH, United Kingdom [Z. P., P. T. S.]; Cancer Research Hodgkin’s lymphoma, and acute leukemia (3). Intercalation is UK Department of Medical Oncology, University of Glasgow, Glasgow a crucial part of topo II inhibition by cytotoxic anthraquinones G61 1BD, United Kingdom [J. A. P.]; and School of Cell and Molecular Biosciences, The Medical School, University of Newcastle upon Tyne, with high affinity for DNA (4). It is likely that the potent Newcastle upon Tyne NE2 4HH, United Kingdom [E. W., C. A. A.] cytotoxicity of anthraquinones is related to their slow rate of dissociation from DNA, the kinetics of which favors long- term trapping of the topo-DNA complexes (5). However, Abstract currently available DNA intercalators at best promote a tran- Chloroethylaminoanthraquinones are described with sient inhibition of topo II, because the topo-drug-DNA ter- intercalating and alkylating capacity that potentially nary complex is reversed by removal of the intracellular drug covalently cross-link topoisomerase II (topo II) to DNA. -

5-Fluorouracil + Adriamycin + Cyclophosphamide) Combination in Differentiated H9c2 Cells
Article Doxorubicin Is Key for the Cardiotoxicity of FAC (5-Fluorouracil + Adriamycin + Cyclophosphamide) Combination in Differentiated H9c2 Cells Maria Pereira-Oliveira, Ana Reis-Mendes, Félix Carvalho, Fernando Remião, Maria de Lourdes Bastos and Vera Marisa Costa * UCIBIO, REQUIMTE, Laboratory of Toxicology, Faculty of Pharmacy, University of Porto, Rua de Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal; [email protected] (M.P.-O.); [email protected] (A.R.-M.); [email protected] (F.C.); [email protected] (F.R.); [email protected] (M.L.B.) * Correspondence: [email protected] Received: 4 October 2018; Accepted: 3 January 2019; Published: 10 January 2019 Abstract: Currently, a common therapeutic approach in cancer treatment encompasses a drug combination to attain an overall better efficacy. Unfortunately, it leads to a higher incidence of severe side effects, namely cardiotoxicity. This work aimed to assess the cytotoxicity of doxorubicin (DOX, also known as Adriamycin), 5-fluorouracil (5-FU), cyclophosphamide (CYA), and their combination (5-Fluorouracil + Adriamycin + Cyclophosphamide, FAC) in H9c2 cardiac cells, for a better understanding of the contribution of each drug to FAC-induced cardiotoxicity. Differentiated H9c2 cells were exposed to pharmacological relevant concentrations of DOX (0.13–5 μM), 5-FU (0.13–5 μM), CYA (0.13–5 μM) for 24 or 48 h. Cells were also exposed to FAC mixtures (0.2, 1 or 5 μM of each drug and 50 μM 5-FU + 1 μM DOX + 50 μM CYA). DOX was the most cytotoxic drug, followed by 5-FU and lastly CYA in both cytotoxicity assays (reduction of 3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyl tetrazolium bromide (MTT) and neutral red (NR) uptake). -

Tasigna, INN-Nilotinib
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Tasigna 50 mg hard capsules Tasigna 200 mg hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tasigna 50 mg hard capsules One hard capsule contains 50 mg nilotinib (as hydrochloride monohydrate). Excipient with known effect One hard capsule contains 39.03 mg lactose monohydrate. Tasigna 200 mg hard capsules One hard capsule contains 200 mg nilotinib (as hydrochloride monohydrate). Excipient with known effect One hard capsule contains 156.11 mg lactose monohydrate. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Hard capsule. Tasigna 50 mg hard capsules White to yellowish powder in hard gelatin capsule with red opaque cap and light yellow opaque body, size 4 with black radial imprint “NVR/ABL” on cap. Tasigna 200 mg hard capsules White to yellowish powder in light yellow opaque hard gelatin capsules, size 0 with red axial imprint “NVR/TKI”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Tasigna is indicated for the treatment of: - adult and paediatric patients with newly diagnosed Philadelphia chromosome positive chronic myelogenous leukaemia (CML) in the chronic phase, - adult patients with chronic phase and accelerated phase Philadelphia chromosome positive CML with resistance or intolerance to prior therapy including imatinib. Efficacy data in patients with CML in blast crisis are not available, - paediatric patients with chronic phase Philadelphia chromosome positive CML with resistance or intolerance to prior therapy including imatinib. 2 4.2 Posology and method of administration Therapy should be initiated by a physician experienced in the diagnosis and the treatment of patients with CML. -

Thames Valley Chemotherapy Regimens Sarcoma
Thames Valley Thames Valley Chemotherapy Regimens Sarcoma Chemotherapy Regimens– Sarcoma 1 of 98 Thames Valley Notes from the editor All chemotherapy regimens, and associated guidelines eg antiemetics and dose bands are available on the Network website www.tvscn.nhs.uk/networks/cancer-topics/chemotherapy/ Any correspondence about the regimens should be addressed to: Sally Coutts, Cancer Pharmacist, Thames Valley email: [email protected] Acknowledgements These regimens have been compiled by the Network Pharmacy Group in collaboration with key contribution from Prof Bass Hassan, Medical Oncologist, OUH Dr Sally Trent, Clinical Oncologist, OUH Dr James Gildersleve, Clinical Oncologist, RBFT Dr Sarah Pratap, Medical Oncologist, OUH Dr Shaun Wilson, TYA - Paediatric Oncologist, OUH Catherine Chaytor, Cancer Pharmacist, OUH Varsha Ormerod, Cancer Pharmacist, OUH Kristen Moorhouse, Cancer Pharmacist, OUH © Thames Valley Cancer Network. All rights reserved. Not to be reproduced in whole or in part without the permission of the copyright owner. Chemotherapy Regimens– Sarcoma 2 of 98 Thames Valley Thames Valley Chemotherapy Regimens Sarcoma Network Chemotherapy Regimens used in the management of Sarcoma Date published: January 2019 Date of review: June 2022 Chemotherapy Regimens Name of regimen Indication Page List of amendments to this version 5 Imatinib GIST 6 Sunitinib GIST 9 Regorafenib GIST 11 Paclitaxel weekly (Taxol) Angiosarcoma 13 AC Osteosarcoma 15 Cisplatin Imatinib – if local Trust funding agreed Chordoma 18 Doxorubicin Sarcoma 21 -

The Effects of Combination Treatments on Drug Resistance in Chronic Myeloid Leukaemia: an Evaluation of the Tyrosine Kinase Inhibitors Axitinib and Asciminib H
Lindström and Friedman BMC Cancer (2020) 20:397 https://doi.org/10.1186/s12885-020-06782-9 RESEARCH ARTICLE Open Access The effects of combination treatments on drug resistance in chronic myeloid leukaemia: an evaluation of the tyrosine kinase inhibitors axitinib and asciminib H. Jonathan G. Lindström and Ran Friedman* Abstract Background: Chronic myeloid leukaemia is in principle a treatable malignancy but drug resistance is lowering survival. Recent drug discoveries have opened up new options for drug combinations, which is a concept used in other areas for preventing drug resistance. Two of these are (I) Axitinib, which inhibits the T315I mutation of BCR-ABL1, a main source of drug resistance, and (II) Asciminib, which has been developed as an allosteric BCR-ABL1 inhibitor, targeting an entirely different binding site, and as such does not compete for binding with other drugs. These drugs offer new treatment options. Methods: We measured the proliferation of KCL-22 cells exposed to imatinib–dasatinib, imatinib–asciminib and dasatinib–asciminib combinations and calculated combination index graphs for each case. Moreover, using the median–effect equation we calculated how much axitinib can reduce the growth advantage of T315I mutant clones in combination with available drugs. In addition, we calculated how much the total drug burden could be reduced by combinations using asciminib and other drugs, and evaluated which mutations such combinations might be sensitive to. Results: Asciminib had synergistic interactions with imatinib or dasatinib in KCL-22 cells at high degrees of inhibition. Interestingly, some antagonism between asciminib and the other drugs was present at lower degrees on inhibition. -

Bridging from Preclinical to Clinical Studies for Tyrosine Kinase Inhibitors Based on Pharmacokinetics/Pharmacodynamics and Toxicokinetics/Toxicodynamics
Drug Metab. Pharmacokinet. 26 (6): 612620 (2011). Copyright © 2011 by the Japanese Society for the Study of Xenobiotics (JSSX) Regular Article Bridging from Preclinical to Clinical Studies for Tyrosine Kinase Inhibitors Based on Pharmacokinetics/Pharmacodynamics and Toxicokinetics/Toxicodynamics Azusa HOSHINO-YOSHINO1,2,MotohiroKATO2,KohnosukeNAKANO2, Masaki ISHIGAI2,ToshiyukiKUDO1 and Kiyomi ITO1,* 1Research Institute of Pharmaceutical Sciences, Musashino University, Tokyo, Japan 2Pre-clinical Research Department, Chugai Pharmaceutical Co. Ltd., Kanagawa, Japan Full text of this paper is available at http://www.jstage.jst.go.jp/browse/dmpk Summary: The purpose of this study was to provide a pharmacokinetics/pharmacodynamics and toxi- cokinetics/toxicodynamics bridging of kinase inhibitors by identifying the relationship between their clinical and preclinical (rat, dog, and monkey) data on exposure and efficacy/toxicity. For the eight kinase inhibitors approved in Japan (imatinib, gefitinib, erlotinib, sorafenib, sunitinib, nilotinib, dasatinib, and lapatinib), the human unbound area under the concentration-time curve at steady state (AUCss,u) at the clinical dose correlated well with animal AUCss,u at the no-observed-adverse-effect level (NOAEL) or maximum tolerated dose (MTD). The best correlation was observed for rat AUCss,u at the MTD (p < 0.001). Emax model analysis was performed using the efficacy of each drug in xenograft mice, and the efficacy at the human AUC of the clinical dose was evaluated. The predicted efficacy at the human AUC of the clinical dose varied from far below Emax to around Emax even in the tumor for which use of the drugs had been accepted. These results suggest that rat AUCss,u attheMTD,butnottheefficacy in xenograft mice, may be a useful parameter to estimate the human clinical dose of kinase inhibitors, which seems to be currently determined by toxicity rather than efficacy. -

Cardiotoxicity of Doxorubicin Is Mediated Through Mitochondrial Iron Accumulation
Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa, … , Tejaswitha Jairaj Naik, Hossein Ardehali J Clin Invest. 2014;124(2):617-630. https://doi.org/10.1172/JCI72931. Research Article Cardiology Doxorubicin is an effective anticancer drug with known cardiotoxic side effects. It has been hypothesized that doxorubicin- dependent cardiotoxicity occurs through ROS production and possibly cellular iron accumulation. Here, we found that cardiotoxicity develops through the preferential accumulation of iron inside the mitochondria following doxorubicin treatment. In isolated cardiomyocytes, doxorubicin became concentrated in the mitochondria and increased both mitochondrial iron and cellular ROS levels. Overexpression of ABCB8, a mitochondrial protein that facilitates iron export, in vitro and in the hearts of transgenic mice decreased mitochondrial iron and cellular ROS and protected against doxorubicin-induced cardiomyopathy. Dexrazoxane, a drug that attenuates doxorubicin-induced cardiotoxicity, decreased mitochondrial iron levels and reversed doxorubicin-induced cardiac damage. Finally, hearts from patients with doxorubicin-induced cardiomyopathy had markedly higher mitochondrial iron levels than hearts from patients with other types of cardiomyopathies or normal cardiac function. These results suggest that the cardiotoxic effects of doxorubicin develop from mitochondrial iron accumulation and that reducing mitochondrial iron levels protects against doxorubicin- induced cardiomyopathy. Find the latest version: https://jci.me/72931/pdf Research article Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa,1 Mohsen Ghanefar,1 Marina Bayeva,1 Rongxue Wu,1 Arineh Khechaduri,1 Sathyamangla V. Naga Prasad,2 R. Kannan Mutharasan,1 Tejaswitha Jairaj Naik,1 and Hossein Ardehali1 1Feinberg Cardiovascular Institute, Northwestern University School of Medicine, Chicago, Illinois, USA. -

ZINECARD® (Dexrazoxane) for Injection Regimens
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------DOSAGE FORMS AND STRENGTHS------------- These highlights do not include all the information needed to use 250 mg or 500 mg single dose vials as sterile, pyrogen-free lyophilizates. (3) ZINECARD safely and effectively. See full prescribing information for ZINECARD. -------------------------------CONTRAINDICATIONS------------------------------ ZINECARD should not be used with non-anthracycline chemotherapy ZINECARD® (dexrazoxane) for injection regimens. (4) Initial U.S. Approval: 1995 -----------------------WARNINGS AND PRECAUTIONS------------------------ ---------------------------INDICATIONS AND USAGE----------------------- Myelosuppression: ZINECARD may increase the myelosuppresive ZINECARD is a cytoprotective agent indicated for reducing the incidence and effects of chemotherapeutic agents. Perform hematological monitoring. severity of cardiomyopathy associated with doxorubicin administration in (5.1) women with metastatic breast cancer who have received a cumulative Embryo-Fetal Toxicity: Can cause fetal harm. Advise female patients of doxorubicin dose of 300 mg/m2 and who will continue to receive doxorubicin reproductive potential of the potential hazard to the fetus. (5.5, 8.1) therapy to maintain tumor control. Do not use ZINECARD with doxorubicin initiation. (1) ------------------------------ADVERSE REACTIONS------------------------------- In clinical studies, ZINECARD was administered to patients also receiving -----------------------DOSAGE AND ADMINISTRATION---------------------- chemotherapeutic agents for cancer. Pain on injection was observed more Reconstitute vial contents and dilute before use. (2.3) frequently in patients receiving ZINECARD versus placebo. (6.1) Administer ZINECARD by intravenous infusion over 15 minutes. DO NOT ADMINISTER VIA AN INTRAVENOUS PUSH. (2.1, 2.3) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at The recommended dosage ratio of ZINECARD to doxorubicin is 10:1 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

Cardioprotective Effects of Exercise Training on Doxorubicin-Induced
www.nature.com/scientificreports OPEN Cardioprotective efects of exercise training on doxorubicin‑induced cardiomyopathy: a systematic review with meta‑analysis of preclinical studies Paola Victória da Costa Ghignatti, Laura Jesuíno Nogueira, Alexandre Machado Lehnen & Natalia Motta Leguisamo* Doxorubicin (DOX)‑induced cardiotoxicity in chemotherapy is a major treatment drawback. Clinical trials on the cardioprotective efects of exercise in cancer patients have not yet been published. Thus, we conducted a systematic review and meta‑analysis of preclinical studies for to assess the efcacy of exercise training on DOX‑induced cardiomyopathy. We included studies with animal models of DOX‑induced cardiomyopathy and exercise training from PubMed, Web of Sciences and Scopus databases. The outcome was the mean diference (MD) in fractional shortening (FS, %) assessed by echocardiography between sedentary and trained DOX‑treated animals. Trained DOX‑treated animals improved 7.40% (95% CI 5.75–9.05, p < 0.001) in FS vs. sedentary animals. Subgroup analyses revealed a superior efect of exercise training execution prior to DOX exposure (MD = 8.20, 95% CI 6.27–10.13, p = 0.010). The assessment of cardiac function up to 10 days after DOX exposure and completion of exercise protocol was also associated with superior efect size in FS (MD = 7.89, 95% CI 6.11–9.67, p = 0.020) vs. an echocardiography after over 4 weeks. Modality and duration of exercise, gender and cumulative DOX dose did were not individually associated with changes on FS. Exercise training is a cardioprotective approach in rodent models of DOX‑induced cardiomyopathy. Exercise prior to DOX exposure exerts greater efect sizes on FS preservation. -
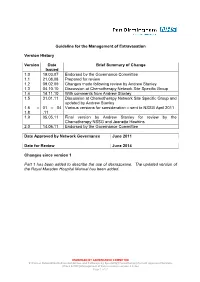
Guideline for the Management of Extravasation
Guideline for the Management of Extravasation Version History Version Date Brief Summary of Change Issued 1.0 19.03.07 Endorsed by the Governance Committee 1.1 21.08.08 Prepared for review 1.2 09.02.09 Changes made following review by Andrew Stanley 1.3 04.10.10 Discussion at Chemotherapy Network Site Specific Group 1.4 14.11.10 With comments from Andrew Stanley 1.5 31.01.11 Discussion at Chemotherapy Network Site Specific Group and updated by Andrew Stanley 1.6 – 01 – 04 Various versions for consideration – sent to NSSG April 2011 1.8 .11 1.9 05.05.11 Final version by Andrew Stanley for review by the Chemotherapy NSSG and Jeanette Hawkins 2.0 14.06.11 Endorsed by the Governance Committee Date Approved by Network Governance June 2011 Date for Review June 2014 Changes since version 1 Part 1 has been added to describe the use of dexrazoxane. The updated version of the Royal Marsden Hospital Manual has been added. ENDORSED BY GOVERNANCE COMMITTEE S:\Cancer Network\Guidelines\Guidelines and Pathways by Speciality\Chemotherapy\Current Approved Versions (Word & PDF)\Management of Extravasation version 2.0.doc Page 1 of 21 1 Scope of the Guideline This guidance has been produced to support the following: The prevention of the extravasation of intravenous anti-cancer drugs. The early detection of the extravasation of intravenous anti-cancer drugs. The treatment of the extravasation of intravenous anti-cancer drugs. 2 Guideline Statement Statement 2 The Network Site Specific Group has agreed to adopt the Royal Marsden Hospital Manual of Clinical Nursing Procedures 7th Edition; Blackwell Publishing (2008), chapter on extravasation, with the addition of a section on dexrazoxane.