A Glossary for Mass Spectrometry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

About Magnetic Sector Mass Spec- Trometers
About Magnetic Sector Mass Spec- trometers Double-focusing magnetic sector mass spectrometers provide high sensitivity, high resolution, and a reproducibility that is unmatched in any other kind of mass analyzer. A double-focusing magnetic sector mass spectrometer has at least four components: . An ion source in which ions are formed and accelerated to energies of up to as high as 10 kilovolts. A magnetic sector with a magnetic field that exerts a force perpendicular to the ion mo- tion to deflect ions according to their momentum. An electric sector with an electric field that exerts a force perpendicular to the ion mo- tion to deflect ions according to their kinetic energy. A detector that produces a response that is proportional to the number of ions. Slits are placed in the ion path to define the positions and energies of the ions that strike the detector. In general, decreasing the slit widths increases the mass resolution but re- duces the number of ions that are detected. Additional electrostatic lenses are com- monly used to shape and deflect the ion beam to optimize peak shape and maximize ion beam transmission from the source to the detector. JEOL uses octapole and quadrupole focusing lenses to simplify the ion optical design. Collision chambers in the first field-free region (just after the ion source) and second field-free region (just after the magnet and before the electric sector) are used to induce ions to fragment in collision-induced-dissociation experiments (MS/MS). The term "double-focusing" refers to the fact that the combination of electric and mag- netic sectors focuses ions according to both direction and energy to provide higher reso- lution than can be obtained with a single magnetic sector. -

Mass Spectrometer Detectors
Mass Spectrometer Detectors www.sge.com Ion Detectors for Virtually all Mass Spectrometry Applications Electron multipliers are used in a wide range of applications to Figure 2. Secondary Electron Emission detect and measure small signals of ions, electrons or photons. One of the main applications for an electron multiplier is for 6 the detection of ions in a mass spectrometer. ACTIVE FILM Multipliers manufactured by ETP ( a division of SGE Group 5 of Companies) are the result of 15 years experience in 4 supplying high performance electron multipliers for use in mass spectrometry applications. The performance of this 3 unique type of ion detector has seen it become widely used in almost all fields of mass spectrometry (including ICP-MS, 2 GC-MS, LC-MS, TOF and SIMS). 1 An electron multiplier is used to detect the presence of ion Secondary Electron Emissions signals emerging from the mass analyser of a mass 0 spectrometer. It is essentially the "eyes" of the instrument (see 0 100 200 300 400 500 Figure 1). The task of the electron multiplier is to detect Incident Electron Energy (eV) every ion of the selected mass passed by the mass filter. How The average number of secondary electrons emitted from the efficiently the electron multiplier carries out this task surface of an ETP electron multiplier plotted against the represents a potentially limiting factor on the overall system energy of the incident primary electron. sensitivity. Consequently the performance of the electron multiplier can have a major influence on the overall (often referred to as a channel electron multiplier or CEM). -

Microchannel Plate Detectors
Reprinted from Nuclear Instruments and Methods, Vol. 162, 1979, pages 587 to 601 MICROCHANNEL PLATE DETECTORS JOSEPH LADISLAS WIZA 1. Introduction typical. Originally developed as an amplification element for A microchannel plate (MCP) is an array of 104-107 image intensification devices, MCPs have direct sensitivity miniature electron multipliers oriented parallel to one to charged particles and energetic photons which has another (fig. 1); typical channel diameters are in the range extended their usefulness to such diverse fields as X-ray1) 10-100 µm and have length to diameter ratios (α) between and E.U.V.2) astronomy, e-beam fusion studies3) and of 40 and 100. Channel axes are typically normal to, or biased course, nuclear science, where to date most applications at a small angle (~8°) to the MCP input surface. The have capitalized on the superior MCP time resolution channel matrix is usually fabricated from a lead glass, characteristics4-6). treated in such a way as to optimize the secondary emission The MCP is the result of a fortuitous convergence of characteristics of each channel and to render the channel technologies. The continuous dynode electron multiplier walls semiconducting so as to allow charge replenishment was suggested by Farnsworth7) in 1930. Actual from an external voltage source. Thus each channel can be implementation, however, was delayed until the 1960s considered to be a continuous dynode structure which acts when experimental work by Oschepkov et al.8) from the as its own dynode resistor chain. Parallel electrical contact USSR, Goodrich and Wiley9) at the Bendix Research to each channel is provided by the deposition of a metallic Laboratories in the USA, and Adams and Manley10-11) at coating, usually Nichrome or Inconel, on the front and rear the Mullard Research Laboratories in the U.K. -

Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatograph
applied sciences Article Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatography Mass Spectrometry, Electrospray and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry Bo-Hong Chen, Ju-Tsung Liu, Hung-Ming Chen, Wen-Xiong Chen and Cheng-Huang Lin * Department of Chemistry, National Taiwan Normal University, 88 Sec. 4 Tingchow Road, Taipei 11677, Taiwan; [email protected] (B.-H.C.); [email protected] (J.-T.L.); [email protected] (H.-M.C.); [email protected] (W.-X.C.) * Correspondence: [email protected]; Tel.: +886-2-7734-6170; Fax: +886-2-2932-4249 Received: 18 April 2018; Accepted: 19 June 2018; Published: 22 June 2018 Abstract: Characteristic mass fragmentation of 20 phenethylamine/tryptamine standards were investigated and compared by means of matrix assisted laser desorption/time-of-flight mass spectrometry (MALDI/TOFM), gas chromatography–electron ionization–mass spectrometry (GC-EI/MS) and liquid chromatography–electrospray ionization/mass spectrometry (LC-ESI/MS) + methods. As a result, three characteristic peaks ([M] and fragments from the Cβ-Cα bond breakage) were found to be unique and contained information useful in identifying 2C series compounds based on the GC-EI/MS method. We found that the protonated molecular ion ([M+H]+) and two types of fragments produced from the α-cleavage and β-cleavage processes were useful mass spectral information in the rapid screening and confirmation of phenethylamine and tryptamine derivatives when ESI/MS and MALDI/TOFMS methods were applied. This assay was successfully used to determine samples that contain illicit drugs. Keywords: phenethylamine; tryptamine; MALDI/TOFMS; GC-EI/MS; LC-ESI/MS 1. -

Coupling Gas Chromatography to Mass Spectrometry
Coupling Gas Chromatography to Mass Spectrometry Introduction The suite of gas chromatographic detectors includes (roughly in order from most common to the least): the flame ionization detector (FID), thermal conductivity detector (TCD or hot wire detector), electron capture detector (ECD), photoionization detector (PID), flame photometric detector (FPD), thermionic detector, and a few more unusual or VERY expensive choices like the atomic emission detector (AED) and the ozone- or fluorine-induce chemiluminescence detectors. All of these except the AED produce an electrical signal that varies with the amount of analyte exiting the chromatographic column. The AED does that AND yields the emission spectrum of selected elements in the analytes as well. Another GC detector that is also very expensive but very powerful is a scaled down version of the mass spectrometer. When coupled to a GC the detection system itself is often referred to as the mass selective detector or more simply the mass detector. This powerful analytical technique belongs to the class of hyphenated analytical instrumentation (since each part had a different beginning and can exist independently) and is called gas chromatograhy/mass spectrometry (GC/MS). Placed at the end of a capillary column in a manner similar to the other GC detectors, the mass detector is more complicated than, for instance, the FID because of the mass spectrometer's complex requirements for the process of creation, separation, and detection of gas phase ions. A capillary column is required in the chromatograph because the entire MS process must be carried out at very low pressures (~10-5 torr) and in order to meet this requirement a vacuum is maintained via constant pumping using a vacuum pump. -

Characterization of Morphological and Chemical Properties of Scandium Containing
Characterization of Morphological and Chemical Properties of Scandium Containing Cathode Materials A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Michael V. Mroz May 2020 © 2020 Michael V. Mroz. All Rights Reserved. 2 This dissertation titled Characterization of Morphological and Chemical Properties of Scandium Containing Cathode Materials by MICHAEL V. MROZ has been approved for the Department of Physics and Astronomy and the College of Arts and Sciences by Martin E. Kordesch Professor of Physics and Astronomy Florenz Plassmann Dean, College of Arts and Sciences 3 ABSTRACT MROZ, MICHAEL V., Ph.D., May 2020, Physics and Astronomy Characterization of Morphological and Chemical Properties of Scandium Containing Cathode Materials Director of Dissertation: Martin E. Kordesch Understanding thermionic cathodes is crucial for the future development of communication technologies operating at the terahertz frequency. Model cathode systems were characterized using multiple experimental techniques. These included Low Energy Electron Microscopy, X-Ray Photoemission Spectroscopy, and Auger Electron Spectroscopy. This was done to determine the mechanisms by which tungsten, barium, scandium, and oxygen may combine in order to achieve high current densities via thermionic emission. Barium and scandium films are found to dewet from the tungsten surfaces studied, and not diffuse out from bulk sources. The dewetted droplets were found to contribute the most to thermal emission. Barium oxide and scandium oxide are also found to react desorb from the emitting surface at lower temperatures then the metals themselves. The function of scandium in scandate cathodes was determined to act as an inhibitor to oxide formation. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -

Modern Mass Spectrometry
Modern Mass Spectrometry MacMillan Group Meeting 2005 Sandra Lee Key References: E. Uggerud, S. Petrie, D. K. Bohme, F. Turecek, D. Schröder, H. Schwarz, D. Plattner, T. Wyttenbach, M. T. Bowers, P. B. Armentrout, S. A. Truger, T. Junker, G. Suizdak, Mark Brönstrup. Topics in Current Chemistry: Modern Mass Spectroscopy, pp. 1-302, 225. Springer-Verlag, Berlin, 2003. Current Topics in Organic Chemistry 2003, 15, 1503-1624 1 The Basics of Mass Spectroscopy ! Purpose Mass spectrometers use the difference in mass-to-charge ratio (m/z) of ionized atoms or molecules to separate them. Therefore, mass spectroscopy allows quantitation of atoms or molecules and provides structural information by the identification of distinctive fragmentation patterns. The general operation of a mass spectrometer is: "1. " create gas-phase ions "2. " separate the ions in space or time based on their mass-to-charge ratio "3. " measure the quantity of ions of each mass-to-charge ratio Ionization sources ! Instrumentation Chemical Ionisation (CI) Atmospheric Pressure CI!(APCI) Electron Impact!(EI) Electrospray Ionization!(ESI) SORTING DETECTION IONIZATION OF IONS OF IONS Fast Atom Bombardment (FAB) Field Desorption/Field Ionisation (FD/FI) Matrix Assisted Laser Desorption gaseous mass ion Ionisation!(MALDI) ion source analyzer transducer Thermospray Ionisation (TI) Analyzers quadrupoles vacuum signal Time-of-Flight (TOF) pump processor magnetic sectors 10-5– 10-8 torr Fourier transform and quadrupole ion traps inlet Detectors mass electron multiplier spectrum Faraday cup Ionization Sources: Classical Methods ! Electron Impact Ionization A beam of electrons passes through a gas-phase sample and collides with neutral analyte molcules (M) to produce a positively charged ion or a fragment ion. -

Fundamentals of Biological Mass Spectrometry and Proteomics
Fundamentals of Biological Mass Spectrometry and Proteomics Steve Carr Broad Institute of MIT and Harvard Modern Mass Spectrometer (MS) Systems Orbitrap Q-Exactive Triple Quadrupole Discovery/Global Experiments Targeted MS MS systems used for proteomics have 4 tasks: • Create ions from analyte molecules • Separate the ions based on charge and mass • Detect ions and determine their mass-to-charge • Select and fragment ions of interest to provide structural information (MS/MS) Electrospray MS: ease of coupling to liquid-based separation methods has made it the key technology in proteomics Possible Sample Inlets Syringe Pump Sample Injection Loop Liquid Autosampler, HPLC Capillary Electrophoresis Expansion of the Ion Formation and Sampling Regions Nitrogen Drying Gas Electrospray Atmosphere Vacuum Needle 3- 5 kV Liquid Nebulizing Gas Droplets Ions Containing Solvated Ions Isotopes Most elements have more than one stable isotope. For example, most carbon atoms have a mass of 12 Da, but in nature, 1.1% of C atoms have an extra neutron, making their mass 13 Da. Why do we care? Mass spectrometers “see” the isotope peaks provided the resolution is high enough. If an MS instrument has resolution high enough to resolve these isotopes, better mass accuracy is achieved. Stable isotopes of most abundant elements of peptides Element Mass Abundance H 1.0078 99.985% 2.0141 0.015 C 12.0000 98.89 13.0034 1.11 N 14.0031 99.64 15.0001 0.36 O 15.9949 99.76 16.9991 0.04 17.9992 0.20 Monoisotopic mass and isotopes We use instruments that resolve the isotopes enabling us to accurately measure the monoisotopic mass MonoisotopicMonoisotopic mass; all 12C, mass no 13C atoms corresponds to 13 lowestOne massC atom peak Two 13C atoms Angiotensin I (MW = 1295.6) (M+H)+ = C62 H90 N17 O14 TheWhen monoisotopic the isotopes mass of aare molecule clearly is the resolved sum of the the accurate monoisotopic masses for the massmost abundant isotope of each element present. -
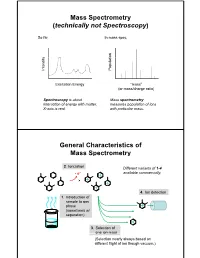
Mass Spectrometry (Technically Not Spectroscopy)
Mass Spectrometry (technically not Spectroscopy) So far, In mass spec, on y Populati Intensit Excitation Energy “mass” (or mass/charge ratio) Spectroscopy is about Mass spectrometry interaction of energy with matter. measures population of ions X-axis is real. with particular mass. General Characteristics of Mass Spectrometry 2. Ionization Different variants of 1-4 -e- available commercially. 4. Ion detection 1. Introduction of sample to gas phase (sometimes w/ separation) 3. Selection of one ion mass (Selection nearly always based on different flight of ion though vacuum.) General Components of a Mass Spectrometer Lots of choices, which can be mixed and matched. direct injection The Mass Spectrum fragment “daughter” ions M+ “parent” mass Sample Introduction: Direc t Inser tion Prob e If sample is a liquid, sample can also be injected directly into ionization region. If sample isn’t pure, get multiple parents (that can’t be distinguished from fragments). Capillary Column Introduction Continous source of molecules to spectrometer. detector column (including GC, LC, chiral, size exclusion) • Signal intensity depends on both amount of molecule and ionization efficiency • To use quantitatively, must calibrate peaks with respect eltilution time ttlitotal ion curren t to quantity eluted (TIC) over time Capillary Column Introduction Easy to interface with gas or liquid chromatography. TIC trace elution time time averaged time averaged mass spectrum mass spectrum Methods of Ionization: Electron Ionization (EI) 1 - + - 1 M + e (kV energy) M + 2e Fragmentation in Electron Ionization daughter ion (observed in spectrum) neutral fragment (not observed) excited parent at electron at electron energy of energy of 15 eV 70 e V Lower electron energy yields less fragmentation, but also less signal. -

Appendix the Meaning and Usage of the Terms Monoisotopic Mass
MASS SPECTROMETRY IN BIOLOGY AND MEDICINE Appendix The Meaning and Usage of the Terms Monoisotopic Mass, Average Mass, Mass Resolution, and Mass Accuracy for Measurements of Biomolecules s. A. Carr, A. L. Burlingame and M. A. Baldwin Much confusion surrounds the meaning of the terms monoisotopic mass, average mass, resolution and mass accuracy in mass spectrometry, and how they interrelate. The following brief primer is intended to clarify these terms and to illustrate the effect they have on the data obtained and its interpretation. We have highlighted the effects of these parameters on measurements of masses spanning the range of 1000 to 25,000 Da because of important differences that are observed. We refer the interested reader to a number ofexcellent articles that have dealt with aspects ofthese issues [1-6]. MONOISOTOPIC MASS VERSUS AVERAGE MASS Most elements have a variety of naturally occurring isotopes, each with a unique mass and natural abundance. The monoisotopic mass of an element refers specifically to the lightest stable isotope of that element. For example, there are two principle isotopes of carbon, l2C and l3C, with masses of 12.000000 and 13.003355 and natural abundances of98.9 and 1.1%, respectively; thus the value defined for the monoisotopic mass of carbon is 12.00000 on this mass scale. Similarly, there are two naturally occurring isotopes for nitrogen, l4N, with a mass of 14.003074 (monoisotopic mass) and a relative abundance of99.6% and l5N, with a mass of 15.000109 and a relative abundance of ca. 0.4%. The monoisotopic mass of a molecule is thus obtained by summing the monoisotopic masses (including the decimal component, referred to as the mass defect) of each element present. -

An Introduction to Mass Spectrometry
An Introduction to Mass Spectrometry by Scott E. Van Bramer Widener University Department of Chemistry One University Place Chester, PA 19013 [email protected] http://science.widener.edu/~svanbram revised: September 2, 1998 © Copyright 1997 TABLE OF CONTENTS INTRODUCTION ........................................................... 4 SAMPLE INTRODUCTION ....................................................5 Direct Vapor Inlet .......................................................5 Gas Chromatography.....................................................5 Liquid Chromatography...................................................6 Direct Insertion Probe ....................................................6 Direct Ionization of Sample ................................................6 IONIZATION TECHNIQUES...................................................6 Electron Ionization .......................................................7 Chemical Ionization ..................................................... 9 Fast Atom Bombardment and Secondary Ion Mass Spectrometry .................10 Atmospheric Pressure Ionization and Electrospray Ionization ....................11 Matrix Assisted Laser Desorption/Ionization ................................ 13 Other Ionization Methods ................................................13 Self-Test #1 ...........................................................14 MASS ANALYZERS .........................................................14 Quadrupole ............................................................15