PIC List R18.Cwk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (19) 11, 3,977,878 Roteman (45) Aug
United States Patent (19) 11, 3,977,878 Roteman (45) Aug. 31, 1976 54 EPOXY RESEN PHOTORESIST WITH 3,522, 194 7/1970 Hada et al........................ 260/47 A ODOFORMAND BISMUTH TRPHENYL 3,708,296 1 / 1973 Schlesinger........................ 96/115 P 3,711,391 lf 1973 Feinberg............................ 96/115 P 75 Inventor: Jerome Roteman, Solon, Ohio 3,720,634 3/1973 Statton ........................... 260/47 EC 73) Assignee: American Can Company, 3,721,617 3/1973 Watt ................................. 260/2 EP Greenwich, Conn. Primary Examiner-Jack P. Brammer 22 Filed: Feb. 26, 1975 Attorney, Agent, or Firm-Robert P. Auber; Ernestine (21) Appl. No.: 553,262 C. Bartlett; George P. Ziehmer Related U.S. Application Data 57 ABSTRACT 62 Division of Ser. No. 369,086, June 1 1, 1973, Pat. No. 3,895,954. Photopolymerizable compositions and processes for photopolymerizing such compositions are provided, 52 U.S. Cl................................ 96/86 P; 96/115 R; said process comprising admixing with said epoxides, 96/ 15 P; 204/159. 18; 204/159.23; photosensitive organohalogen compounds in combina 204/159.24; 96/85; 96/87 R tion with an organometallic compound and thereafter 51 Int. Cl.’....................... G03C 1/94; G03C 1/68 applying energy to the resulting mixture. The or 58 Field of Search............. 96/115 R, 1 15 P, 86 P, ganohalogens decompose to liberate an active catalyst 96/85, 87 R; 204/159.18, 159.23, 159.24; which then serves to initiate polymerization of the ep 260/2 EP, 2 EC, 47 A, 47 C; 427/43 oxide material. The organometallic compound func tions synergistically with the organohalogen to en 56) References Cited hance the film forming properties of the resulting pol UNITED STATES PATENTS ymer and or sensitivity of the polymerizable system. -

2010 Respirator Selection Guide
3M Occupational Health and Environmental Safety Division 2010 Respirator Selection Guide Helping Protectat Work, at Home, Peoplefor Life Table of Contents Respirator Selection Criteria ................................................................................1 • Respirator Program Management • Respirator Fit • Protection Factors • Effects from Skin or Eye Contact • Worker Activity • Location of Hazardous Areas • Respirator Characteristics, Capabilities, and Limitations • General Use Instructions • General Use Limitations Format Explanation ................................................................................................4 • Chemical Name • IDLH Level • Odor Threshold • OEL • Synonyms • Respirator Recommendations • Comments Respirator Filter Definitions ..................................................................................9 • 3M 42 CFR 84 Filters –N-Series Filters –R-Series Filters –P-Series Filters How to Use This Guide ........................................................................................11 Chemical Compound Guide ................................................................................15 Respirator Codes and Descriptions ....................................... inside back cover © 3M 2010. 1 Respirator Selection Criteria The 3M™ Respirator Selection Guide respirators, or the use of this guide, contact reusable respirator, the wearer must obtain a includes a list of chemicals for which your local 3M OH&ESD representative or satisfactory fit as indicated by a qualitative 3M respirators -

Tables of Molecular Vibrational Frequencies, Consolidated Volume I
NAT L INST. OF STAND & TECH R.I.C. NBS PUBLICATIONS DATE DUE f i tZ x tx AMP \ Q 1998 Demco, Inc. 38-293 OF STANDARDS LIBRARY UNITED STATES DEPARTMENT OF COMMERCE JUL 2 7 1976 762636 Peter G. Peterson, Secretary C, NATIONAL BUREAU OF STANDARDS • Lawrence M. Kushner, Acting Director ( ; :* no ‘ 3 / 9 7^ Tables of Molecular Vibrational Frequencies Consolidated Volume I Takehiko Shimanouchi Department of Chemistry Faculty of Science University of Tokyo Tokyo, Japan NSRDS-NBS 39 Stand. Ref. Data Ser., Nat. Bur. Stand. (U.S.), 39, 164 pages (June 1972) L NSRDAP © 1972 by the Secretary of Commerce on Behalf of the United States Government Supersedes and extends the data contained in Tables of Molecular Vibrational Frequencies, NSRDS-NBS-6, Part 1; NSRDS-NBS-11, Part 2; and NSRDS-NBS-17, Part 3. Issued June 1972 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 (Order by SD Catalog No. C13. 48:39). Price S3 Stock Number 0303—0845 Library of Congress Catalog Card Number: 66-60085 II Foreword The National Standard Reference Data System provides effective access to the quantitative data of physical science, critically evaluated and compiled for convenience, and readily accessible through a variety of distribution channels. The System was established in 1963 by action of the President’s Office of Science and Technology and the Federal Council for Science and Technology, with responsibility to administer it assigned to the National Bureau of Standards. The System now comprises a complex of data centers and other activities, carried on in academic institutions and other laboratories both in and out of government. -
C1-C4 Halogenated HC Info 16FEB2018.Xlsx
C1-C4 Halogenated Hydrocarbons/Halocarbons Not Otherwise Listed (C1-C4 NOL) Feb 28, 2018 Administrative Council Mtg - Printed 2/16/20184:11 PM Reported Uses (SOURCES:HAZMAP, NJDHSS, Wikipedia, TSCA CDR, mfr literature) TURA TSCA CDR Chemical CAS/Chemica chemical name chemical fire 2015 Chemical Name Pesti- blowing feedstock / supressant / propell- Inventory? TRI? List l Number (synonyms) formula Solvent refriger-ant etchant Other Tier II cide agent intermed-iate flame ant status* retardant insulation for refrigerators, freezers, commercial refrigeration equipment, refrigerated containers and LNG ships; spray foam insulation; insulated metal panels; slabstock and molded flexible foam; refrigerant for chillers; and solvents for metal cleaning and electronics, and circuit flush. https://www.honeywell-blowingagents.com/?document=solstice-lba-technical- HFO-1233zd (E) brochure&download=1 Hydro-fluoro-olefin C H ClF YYY yhttps://www.honeywell-refrigerants.com/americas/product/solstice-zd/ (HFO) 3 2 3 CDR 2016 Honeywell: 100% as propellants and blowing agents for plastics product mfg; R-1233zd CDR Honeywell Solstice® ZD refrigerant, Solstice® 1233zdE, Solstice Blowing Agent; Arkema (current Forane® 1233zd blowing agent for spray PU foam, appliance insulation, etc. and solvent (may Honeywell not be available in US) product) C1-C4 Cat 102687-65-0 1-Chloro-3,3,3-trifluoropropene Y 106‐95‐6 Allyl bromide 2-Propenyl bromide C3H5Br Y y Manufacture of synthetic perfumes, other allyl compounds; Insecticidal fumigant; 1-Propene, 3-bromo- Chemical intermediate in organic synthesis, for resins (copolymer with sulfur dioxide) and fragrances; As a fumigant (if quite volatile) or as a contact poison; C1-C4 Cat Used in the manufacture of plastics and dyestuff. -

United States Patent [191 [Ill 4,094,943 Howarth Et Al
United States Patent [191 [Ill 4,094,943 Howarth et al. [45] June 13, 1978 PROCESS FOR SPINNING WE 2,927,098 3/1960 Goldberg ..................... 2W77.5 AP RETARDANT ELASTOMERIC 3,055,948 9/1962 Hooh et al. .................. 260/77.5 AP COMPOSITIONS 3,115,384 12/1963 Cacella et al. ....................... 264/184 3,275,578 9/1968 Pedijac et al. ............... 260/45.7 RL Inventors: James C. Fletcher, Administrator of 3,347,822 10/1967 Jenkner ........................ 260/45.7 RL the National Aeronautics and Space 3,689,440 9/1972 Glassmann ................... 260/45.7 RL Administration, with respect to an 3,717,614 2/1973 Lyssy et al. ................... 260/77.5 SP invention of John T. Howarth, 3,723,392 3/1973 Konig et al. .................. 2W77.5 SP Reading; Suresh Sheth, Somerville; 3,852,401 12/1974 Suzuki et al. ........................ 264/184 Kenneth R. Sidman, Wayland; 3,855,382 12/1974 Takeya et al. ....................... 264/182 Arthur A. Massucco, Natick, all of 3,867,336 2/1975 Zox .............................. 260/45.7 RL 3,891,599 6/1975 Marciniak et al. ........... 260/45.7 RL Mass. Primary Examiner-Jay H. Woo Appl. No.: 657,998 Attorney, Agent, or Firm-Russell E. Schlorff; John R. Filed: Feb. 13,1976 Manning; Marvin F. Matthews 1571 ABSTRACT Related Application Data U.S. Flame retardant elastomeric compositions comprised of Division of Ser. No. 374,421, Jun. 28, 1973, Pat. No. either spandex type polyurethane having incorporated 3,956,233. into the polymer chain halogen containing polyols, Int. (3.2 ............................................. B29H 21/04 conventional spandex type polyurethanes in physical U.S. -

2021 Chemical Selection Guide
® 2021 CHEMICAL SELECTION GUIDE WARNING The information in the 2021 Moldex Chemical Selection Guide is dated and was accurate to the best of Moldex’s knowledge as of January 2021. This 2021 Guide supercedes all previous Guides, including printed and electronic versions. If you have an electronic version other than the 2021 Guide please delete it from your computer. The 2021 Guide can be accessed online at http://www.moldex.com/pdf/datasheets/chemicalselectionguide.pdf Before selecting Moldex respirators for use, it is important that you refer to the most recent Guide available. If you have any questions on how to use this guide or on the selection and use of any respiratory protection device, call the Moldex Technical Services Department at +1 (800) 421-0668, +1 (310) 837-6500, ext. 512/550 or [email protected]. Products listed in this Guide are subject to the Moldex limited warranty located on the back cover. The user must use the NIOSH multi-vapor program in conjunction with this guide to determine the service life of cartridges. TERMS OF USE “The information contained in this guide are only guidelines. It is the user’s responsibility to make a respirator equipment selection based on factors only known to the user including, but not limited to worksite specific information, air sampling, warning properties, change out schedules and any other criteria that may affect the health and safety of a worker. If you are uncertain as to the appropriateness of a particular device for a specific situation, do not use OR ALLOW USE OF any Moldex –Metric Inc. -

Tables of Molecular Vibrational Frequencies, Consolidated Volume I
NAT L INST. OF STAND & TECH R.I.C. NBS PUBLICATIONS DATE DUE f i tZ x tx AMP \ Q 1998 Demco, Inc. 38-293 OF STANDARDS LIBRARY UNITED STATES DEPARTMENT OF COMMERCE JUL 2 7 1976 762636 Peter G. Peterson, Secretary C, NATIONAL BUREAU OF STANDARDS • Lawrence M. Kushner, Acting Director ( ; :* no ‘ 3 / 9 7^ Tables of Molecular Vibrational Frequencies Consolidated Volume I Takehiko Shimanouchi Department of Chemistry Faculty of Science University of Tokyo Tokyo, Japan NSRDS-NBS 39 Stand. Ref. Data Ser., Nat. Bur. Stand. (U.S.), 39, 164 pages (June 1972) L NSRDAP © 1972 by the Secretary of Commerce on Behalf of the United States Government Supersedes and extends the data contained in Tables of Molecular Vibrational Frequencies, NSRDS-NBS-6, Part 1; NSRDS-NBS-11, Part 2; and NSRDS-NBS-17, Part 3. Issued June 1972 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 (Order by SD Catalog No. C13. 48:39). Price S3 Stock Number 0303—0845 Library of Congress Catalog Card Number: 66-60085 II Foreword The National Standard Reference Data System provides effective access to the quantitative data of physical science, critically evaluated and compiled for convenience, and readily accessible through a variety of distribution channels. The System was established in 1963 by action of the President’s Office of Science and Technology and the Federal Council for Science and Technology, with responsibility to administer it assigned to the National Bureau of Standards. The System now comprises a complex of data centers and other activities, carried on in academic institutions and other laboratories both in and out of government. -
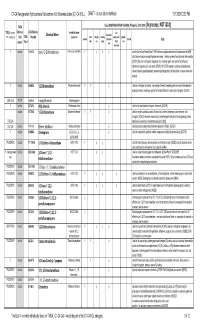
C1-C4 Halogenated Hydrocarbons/Halocarbons Not Otherwise Listed (C1-C4 NOL) DRAFT - Do Not Cite Or Distribute 10/13/20172:39 PM
C1-C4 Halogenated Hydrocarbons/Halocarbons Not Otherwise Listed (C1-C4 NOL) DRAFT - do not cite or distribute 10/13/20172:39 PM TURA Uses (SOURCES:HAZMAP, NJDHSS, Wikipedia, TSCA CDR ) [In process, NOT QCd] TSCA Inventory? Chemica CAS/Chemica chemical name fire Chemical Name feedstock / (Y/N - student ck) l List 2015 l Number (synonyms) Pesti- refriger- blowing supressant / propell- Solvent intermed- etchant Other cide ant agent flame ant status* Tier II iate retardant Y Ind List 156-60-5 trans-1,2-Dichloroethylene trans-Acetylene dichloride Y y Y y used in MicroCare PowerClean II PW2 electronics applications solvent (replacement for nPB) http://www.microcare.com/p-64-powerclean.aspx; Used as a solvent and chemical intermediate; [ACGIH] Used as a refrigerant, degreaser, dry cleaning agent, and solvent for perfumes, adhesives, lacquers, oils, and resins; [HSDB] 2016 CDR reported cleaning and degreasing solvent, blowing agent/propellant, processing/incorporating into formulation, mixture or reaction product Y Ind List 106-93-4 1,2-Dibromoethane Ethylene dibromide Y Y y Used as a fumigant, a solvent, a scavenger for lead in leaded gasoline, and an intermediate in organic synthesis; no longer used in the United States as a soil or grain fumigant; [ACGIH] CDR 2015 Ind List 106-94-5 n-propyl bromide 1-bromopropane Y Ind List 107-05-1 Allyl chloride 1-Chloroprop-2-ene y Used in the manufacture of organic chemicals; [ACGIH] Ind List 107-06-2 1,2-Dichloroethane Ethylene dichloride Y Y y Used in organic synthesis; used in the past as a solvent, -

Dihalocarbene Reactions with Steroid Olefins
AN ABSTRACT OF THE THESIS OF RICHARD HARTMAN CORNELIA for the Ph. D. (Name of student) (Degree) in Chemistry (Organic) presented on March 29, 1968 (Major) (Date) Title: DIHALOCARBENE REACTIONS WITH STEROID OLEFINS Abstract approved: . 1 nomas on Dihalocarbenes have been generated mainly by thermal decom- position of sodium trihaloacetate and phenyl (trihalomethyl)mercury, and give gem -dihalocyclopropyl adducts with various steroid olefins. A new procedure involving the use of sealed tubes makes possible the addition of dihalocarbenes to sterically hindered olefins. In this man- ner, 7- cholestenyl benzoate when treated with PhHgCC13 furnished a 50% yield of the 7a , 8a adduct. Under forcing conditions cholesteryl benzoate was found to undergo an insertion reaction with dichloro- and dibromocarbene to produce 7a -dihalomethyl -5- cholesteryl benzoate. This represents a reospecific dihalocarbene reaction. Dichlorocarbene was found to add, however, to isolated 5, 6 olefins of increased nucleophilicity such as 6- methylcholesteryl acetate. The stereochemistry of the various dihalocyclopropyl adducts has been demonstrated by correlation with compounds of known configuration prepared via stereospecific Simmons -Smith reactions. Both 5a , 6a and 5p, 6p cyclopropyl analogs of cholesterol have been prepared along with other small, strained ring steroids. Dihalocarbene Reactions with Steroid Olefins by Richard Hartman Cornelia A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy June 1968 APPROVED: Assistant Professor of Chemistry in charge of major Chair an of Department o hemistry Dean of Graduate School Date thesis is presented March 29, 1968 Typed by Opal Grossnicklaus for Richard Hartman Cornelia ACKNOWLEDGEMENTS To my wife, Barbara, who has contributed much to the success- ful completion of my graduate study. -

Open PDF File, 2.17 MB, for TURA Reporting Appendices
Toxics Use Reporting Appendices Developed in collaboration with the Office of Technical Assistance and Technology and the Toxics Use Reduction Institute v. 5/14/2020 Contents Appendix A:Standard Industrial Classification (SIC) Codes and North American Industry Classification System (NAICS) Codes Reportable Under the Massachusetts Toxics Use Reduction Act 1 Appendix B: Guidance for TURA Reporting and Planning for Certain Categories and Chemicals ..... 52 Rules for Reporting Water Dissociable Nitrate Compounds Category ......................................................... 52 PBT Chemical and Chemical Category Reporting Thresholds ..................................................................... 53 TURA Higher Hazard Chemicals (1,000 Lb Threshold) .............................................................................. 54 List Of Chemicals For Which A State Only Form R/A Will Be Automatically Generated ......................... 55 Guidance on Reporting Hexavalent Chromium (Cr(VI)) Compounds Use in Combustion and Welding Operations (Higher Hazard Substance, Effective Reporting Year 2012) ..................................................... 56 Guidance on Reporting Formaldehyde as Higher Hazard Substance (Effective Reporting Year 2012) and Quantifying its Use in Combustion Operations ............................................................................................ 61 Guidance on Quantifying Use and Reporting Cyanide Compounds underthe Toxics Use Reduction Act .. 64 Guidance on Reporting Hydrofluoric Acid as a Higher -

Vapor Pressure 6-60
VAPOR PRESSURE This table gives vapor pressure data for about 1800 inorganic and organic substances. In order to accommodate elements and compounds ranging from refractory to highly volatile in a single table, the temperature at which the vapor pressure reaches specified pressure values is listed. The pressure values run in decade steps from 1 Pa (about 7.5 µm Hg) to 100 kPa (about 750 mm Hg). All temperatures are given in °C. The data used in preparing the table came from a large number of sources; the main references used for each substance are indicated in the last column. Since the data were refit in most cases, values appearing in this table may not be identical with values in the source cited. The temperature entry in the 100 kPa column is close to, but not identical with, the normal boiling point (which is defined as the temperature at which the vapor pressure reaches 101.325 kPa). Although some temperatures are quoted to 0.1°C, uncertainties of several degrees should generally be assumed. Values followed by an “e” were obtained by extrapolating (usually with an Antoine equation) beyond the region for which experimental measurements were available and are thus subject to even greater uncertainty. Compounds are listed by molecular formula following the Hill convention. Substances not containing carbon are listed first, followed by those that contain carbon. To locate an organic compound by name or CAS Registry Number when the molecular formula is not known, use the table Physical Constants of Organic Compounds in Section 3 and its indexes to determine the molecular formula.