PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily reflect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scientific institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the first time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -

European Red List of Birds
European Red List of Birds Compiled by BirdLife International Published by the European Commission. opinion whatsoever on the part of the European Commission or BirdLife International concerning the legal status of any country, Citation: Publications of the European Communities. Design and layout by: Imre Sebestyén jr. / UNITgraphics.com Printed by: Pannónia Nyomda Picture credits on cover page: Fratercula arctica to continue into the future. © Ondrej Pelánek All photographs used in this publication remain the property of the original copyright holder (see individual captions for details). Photographs should not be reproduced or used in other contexts without written permission from the copyright holder. Available from: to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed Published by the European Commission. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu). Cataloguing data can be found at the end of this publication. ISBN: 978-92-79-47450-7 DOI: 10.2779/975810 © European Union, 2015 Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Printed in Hungary. European Red List of Birds Consortium iii Table of contents Acknowledgements ...................................................................................................................................................1 Executive summary ...................................................................................................................................................5 1. -

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily re ect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scienti c institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the rst time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -

Biodiversity Assessment for Georgia
Biodiversity Assessment for Georgia Task Order under the Biodiversity & Sustainable Forestry IQC (BIOFOR) USAID C ONTRACT NUMBER: LAG-I-00-99-00014-00 SUBMITTED TO: USAID WASHINGTON E&E BUREAU, ENVIRONMENT & NATURAL RESOURCES DIVISION SUBMITTED BY: CHEMONICS INTERNATIONAL INC. WASHINGTON, D.C. FEBRUARY 2000 TABLE OF CONTENTS SECTION I INTRODUCTION I-1 SECTION II STATUS OF BIODIVERSITY II-1 A. Overview II-1 B. Main Landscape Zones II-2 C. Species Diversity II-4 SECTION III STATUS OF BIODIVERSITY CONSERVATION III-1 A. Protected Areas III-1 B. Conservation Outside Protected Areas III-2 SECTION IV STRATEGIC AND POLICY FRAMEWORK IV-1 A. Policy Framework IV-1 B. Legislative Framework IV-1 C. Institutional Framework IV-4 D. Internationally Supported Projects IV-7 SECTION V SUMMARY OF FINDINGS V-1 SECTION VI RECOMMENDATIONS FOR IMPROVED BIODIVERSITY CONSERVATION VI-1 SECTION VII USAID/GEORGIA VII-1 A. Impact of the Program VII-1 B. Recommendations for USAID/Georgia VII-2 ANNEX A SECTIONS 117 AND 119 OF THE FOREIGN ASSISTANCE ACT A-1 ANNEX B SCOPE OF WORK B-1 ANNEX C LIST OF PERSONS CONTACTED C-1 ANNEX D LISTS OF RARE AND ENDANGERED SPECIES OF GEORGIA D-1 ANNEX E MAP OF LANDSCAPE ZONES (BIOMES) OF GEORGIA E-1 ANNEX F MAP OF PROTECTED AREAS OF GEORGIA F-1 ANNEX G PROTECTED AREAS IN GEORGIA G-1 ANNEX H GEORGIA PROTECTED AREAS DEVELOPMENT PROJECT DESIGN SUMMARY H-1 ANNEX I AGROBIODIVERSITY CONSERVATION IN GEORGIA (FROM GEF PDF GRANT PROPOSAL) I-1 SECTION I Introduction This biodiversity assessment for the Republic of Georgia has three interlinked objectives: · Summarizes the status of biodiversity and its conservation in Georgia; analyzes threats, identifies opportunities, and makes recommendations for the improved conservation of biodiversity. -

National Report on the State of the Environment of Georgia
National Report on the State of the Environment of Georgia 2007 - 2009 FOREWORD This National Report on the State of Environment 2007-2009 has been developed in accordance with the Article 14 of the Law of Georgia on Environmental Protection and the Presidential Decree N 389 of 25 June 1999 on the Rules of Development of National Report on the State of Environment. According to the Georgian legislation, for the purpose of public information the National Report on the State of Environment shall be developed once every three years. 2007-2009 National Report was approved on 9 December 2011. National Report is a summarizing document of all existing information on the state of the environment of Georgia complexly analyzing the state of the environment of Georgia for 2007-2009. The document describes the main directions of environmental policy of the country, presents information on the qualita- tive state of the environment, also presents information on the outcomes of the environmental activities carried out within the frames of international relations, and gives the analysis of environmental impact of different economic sectors. National Report is comprised of 8 Parts and 21 chapters: • Qualitative state of environment (atmospheric air, water resources, land resources, natural disasters, biodiversity, wastes and chemicals, ionizing radiation), • Environmental impact of different economic sectors (agriculture, forestry, transport, industry and en- ergy sector), • Environmental protection management (environmental policy and planning, environmental regula- tion and monitoring, environmental education and awareness raising). In the development of the present State of Environment (SOE) the Ministry of Environment Protection was assisted by the EU funded Project Support to the Improvement of the Environmental Governance in Georgia. -
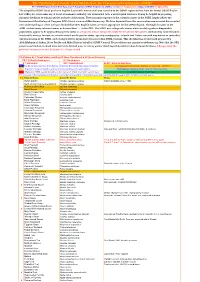
Simplified-ORL-2019-5.1-Final.Pdf
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part F: Simplified OSME Region List (SORL) version 5.1 August 2019. (Aligns with ORL 5.1 July 2019) The simplified OSME list of preferred English & scientific names of all taxa recorded in the OSME region derives from the formal OSME Region List (ORL); see www.osme.org. It is not a taxonomic authority, but is intended to be a useful quick reference. It may be helpful in preparing informal checklists or writing articles on birds of the region. The taxonomic sequence & the scientific names in the SORL largely follow the International Ornithological Congress (IOC) List at www.worldbirdnames.org. We have departed from this source when new research has revealed new understanding or when we have decided that other English names are more appropriate for the OSME Region. The English names in the SORL include many informal names as denoted thus '…' in the ORL. The SORL uses subspecific names where useful; eg where diagnosable populations appear to be approaching species status or are species whose subspecies might be elevated to full species (indicated by round brackets in scientific names); for now, we remain neutral on the precise status - species or subspecies - of such taxa. Future research may amend or contradict our presentation of the SORL; such changes will be incorporated in succeeding SORL versions. This checklist was devised and prepared by AbdulRahman al Sirhan, Steve Preddy and Mike Blair on behalf of OSME Council. Please address any queries to [email protected]. -

The British Birds List of Western Palearctic Birds
The British Birds list of Western Palearctic Birds Struthionidae Ostrich Struthio camelus Anatidae Fulvous Whistling Duck Dendrocygna bicolor Lesser Whistling Duck Dendrocygna javanica Mute Swan Cygnus olor AC2 Black Swan Cygnus atratus Bewick’s Swan Cygnus columbianus A Whooper Swan Cygnus cygnus A Bean Goose Anser fabalis A Pink-footed Goose Anser brachyrhynchus A White-fronted Goose Anser albifrons A Lesser White-fronted Goose Anser erythropus A* Bar-headed Goose Anser indicus Greylag Goose Anser anser AC2C4 Snow Goose Anser caerulescens AC2 Ross’s Goose Anser rossii D* Canada Goose Branta canadensis AC2 Cackling Goose Branta hutchinsii Barnacle Goose Branta leucopsis AC2 Brent Goose Branta bernicla A Red-breasted Goose Branta ruficollis A* Egyptian Goose Alopochen aegyptiaca C1 Ruddy Shelduck Tadorna ferruginea BE Common Shelduck Tadorna tadorna A Spur-winged Goose Plectropterus gambensis Cotton Pygmy-goose Nettapus coromandelianus Wood Duck Aix sponsa Mandarin Duck Aix galericulata C1 Eurasian Wigeon Anas penelope A American Wigeon Anas americana A Falcated Duck Anas falcata D* Gadwall Anas strepera AC2 Baikal Teal Anas formosa A* Eurasian Teal Anas crecca A Green-winged Teal Anas carolinensis A Cape Teal Anas capensis Mallard Anas platyrhynchos AC2C4 Black Duck Anas rubripes A* Pintail Anas acuta A Red-billed Duck Anas erythrorhyncha Garganey Anas querquedula A Blue-winged Teal Anas discors A* Shoveler Anas clypeata A Marbled Duck Marmaronetta angustirostris D* Red-crested Pochard Netta rufina AC2 Southern Pochard Netta erythrophthalma -

Georgia: Eagles and Endemics in Fall
GEORGIA: EAGLES AND ENDEMICS IN FALL 24 SEPTEMBER – 08 OCTOBER 2022 24 SEPTEMBER – 08 OCTOBER 2023 With over one million birds of prey recorded each fall season, including hundreds of thousands of European Honey Buzzards, the Batumi Bottleneck is one of the birding wonders of the world. www.birdingecotours.com [email protected] 2 | ITINERARY Georgia: Eagles and Endemics in Fall We are absolutely delighted to introduce this incredibly exciting bird tour for an exclusive, small group (maximum of eight for a full group, guaranteed departure with just six), to the beautiful country of Georgia. Due to the nature of the birding on this tour, the small group environment will allow for a much better overall experience than that offered in a larger group. Situated on the narrow strip of land between the Black Sea and Caspian Sea, Georgia is the bridge between Europe and Asia. This position results in a stunning array of bird species from both continents, and beyond. Georgia’s bird list stands at 414 species (following International Ornithological Congress (IOC) taxonomy v10.2 in January 2021), impressive for a relatively small country, with many of these birds being highly sought-after by birders from all over the world, due to their localized global distribution or the difficulty associated with accessing them in the rest of their range. Some of the key species that we look for include Caucasian Snowcock, Caspian Snowcock, Caucasian Grouse, Great Rosefinch (Caucasian endemic rubicilla subspecies), and Güldenstädt’s Redstart, often referred to as Georgia’s “Big Five”. Other birds we hope to find during the tour include Bearded Vulture, Dalmatian Pelican, Pallas’s Gull, Armenian Gull, Sociable Lapwing, Alpine Chough, Green Warbler, Mountain Chiffchaff (local lorenzii subspecies and a potential split), Krüper’s Nuthatch, Red-fronted Serin, and Alpine Accentor. -

Birdlife International (2015) European Red List of Birds. Luxembourg: Publications Office of the European Union
European Red List of Birds Compiled by BirdLife International European Red List of Birds Compiled by BirdLife International Published by the European Commission. opinion whatsoever on the part of the European Commission or BirdLife International concerning the legal status of any country, Citation: Publications of the European Communities. Design and layout by: Imre Sebestyén jr. / UNITgraphics.com Printed by: Pannónia Nyomda Picture credits on cover page: Fratercula arctica to continue into the future. © Ondrej Pelánek All photographs used in this publication remain the property of the original copyright holder (see individual captions for details). Photographs should not be reproduced or used in other contexts without written permission from the copyright holder. Available from: to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed Published by the European Commission. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu). Cataloguing data can be found at the end of this publication. ISBN: 978-92-79-47450-7 DOI: 10.2779/975810 © European Union, 2015 Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Printed in Hungary. European Red List of Birds Consortium iii Table of contents Acknowledgements ...................................................................................................................................................1 Executive summary ...................................................................................................................................................5 1. -

Wildlife Issues and Development Prospects in West and Central Asia
FOWECA/TP/9 Forestry Outlook Study for West and Central Asia (FOWECA) Thematic paper Wildlife issues and development prospects in West and Central Asia René Czudek Rome, 2006 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00153 Rome, Italy. © FAO 2006 Wildlife issues and development prospects in West and Central Asia iii TABLE OF CONTENTS BACKGROUND.......................................................................................................................................................V 1 WILDLIFE ISSUES IN THE REGION ...................................................................................................... 1 1.1 CHALLENGES AHEAD .............................................................................................................................. -

Galliforme Guidelines 260409
Guidelines for the Re-introduction of Galliformes for Conservation Purposes Edited by the World Pheasant Association and IUCN/SSC Re-introduction Specialist Group Occasional Paper of the IUCN Species Survival Commission No. 41 IUCN Founded in 1948, IUCN (International Union for the Conservation of Nature) brings together States, government agencies and a diverse range of nongovernmental organizations in a unique world partnership: over 1000 members in all, spread across some 160 countries. As a Union, IUCN seeks to influence, encourage and assist societies throughout the world to conserve the integrity and diversity of nature and to ensure that any use of natural resources is equitable and ecologically sustainable. IUCN builds on the strengths of its members, networks and partners to enhance their capacity and to support global alliances to safeguard natural resources at local, regional and global levels. IUCN Species Survival Commission (SSC) The IUCN Species Survival Commission is a science-based network of close to 8,000 volunteer experts from almost every country of the world, all working together towards achieving the vision of, “A world that values and conserves present levels of biodiversity.” WPA-IUCN SSC Galliformes Specialist Groups The Grouse Specialist Group was established in 1993 when it developed from WPA’s long- standing grouse network. It was designed to be a global voluntary network of individuals involved in the study, conservation, and sustainable management of grouse. The Pheasant Specialist Group was established in 1991 as a voluntary self-help network of scientists, wildlife managers, conservationists, aviculturists and educators. It was particularly concerned with the plight of threatened pheasant species, and ensuring the survival of viable populations in their natural habitats whilst enhancing the quality of human life. -

The British Birds List of Western Palearctic Birds
The British Birds list of Western Palearctic Birds 1 2 3 4 5 6 Struthionidae Ostrich Struthio camelus Anatidae Fulvous Whistling Duck Dendrocygna bicolor Lesser Whistling Duck Dendrocygna javanica Mute Swan Cygnus olor AC2 Black Swan Cygnus atratus Bewick’s Swan Cygnus columbianus A Whooper Swan Cygnus cygnus A Bean Goose Anser fabalis A Pink-footed Goose Anser brachyrhynchus A White-fronted Goose Anser albifrons A Lesser White-fronted Goose Anser erythropus A* Bar-headed Goose Anser indicus Greylag Goose Anser anser AC2C4 Snow Goose Anser caerulescens AC2 Ross’s Goose Anser rossii D* Greater Canada Goose Branta canadensis C2 Lesser Canada Goose Branta hutchinsii Barnacle Goose Branta leucopsis AC2 Brent Goose Branta bernicla A Red-breasted Goose Branta ruficollis A* Egyptian Goose Alopochen aegyptiaca C1 Ruddy Shelduck Tadorna ferruginea BE Common Shelduck Tadorna tadorna A Spur-winged Goose Plectropterus gambensis Cotton Pygmy-goose Nettapus coromandelianus Wood Duck Aix sponsa Mandarin Duck Aix galericulata C1 Eurasian Wigeon Anas penelope A American Wigeon Anas americana A Falcated Duck Anas falcata D* Gadwall Anas strepera AC2 Baikal Teal Anas formosa A* Eurasian Teal Anas crecca A Green-winged Teal Anas carolinensis A Cape Teal Anas capensis Mallard Anas platyrhynchos AC2C4 Black Duck Anas rubripes A* Pintail Anas acuta A Red-billed Duck Anas erythrorhyncha Garganey Anas querquedula A Blue-winged Teal Anas discors A* Shoveler Anas clypeata A Marbled Duck Marmaronetta angustirostris D* Red-crested Pochard Netta rufina AC2 Southern