African Journal of Microbiology Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Côte D'ivoire
CÔTE D’IVOIRE COI Compilation August 2017 United Nations High Commissioner for Refugees Regional Representation for West Africa - RSD Unit UNHCR Côte d’Ivoire UNHCR Regional Representation for West Africa - RSD Unit UNHCR Côte d’Ivoire Côte d’Ivoire COI Compilation August 2017 This report collates country of origin information (COI) on Côte d’Ivoire up to 15 August 2017 on issues of relevance in refugee status determination for Ivorian nationals. The report is based on publicly available information, studies and commentaries. It is illustrative, but is neither exhaustive of information available in the public domain nor intended to be a general report on human-rights conditions. The report is not conclusive as to the merits of any individual refugee claim. All sources are cited and fully referenced. Users should refer to the full text of documents cited and assess the credibility, relevance and timeliness of source material with reference to the specific research concerns arising from individual applications. UNHCR Regional Representation for West Africa Immeuble FAALO Almadies, Route du King Fahd Palace Dakar, Senegal - BP 3125 Phone: +221 33 867 62 07 Kora.unhcr.org - www.unhcr.org Table of Contents List of Abbreviations .............................................................................................................. 4 1 General Information ....................................................................................................... 5 1.1 Historical background ............................................................................................ -
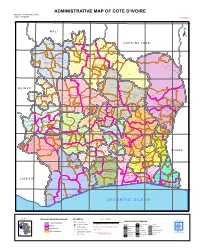
ADMINISTRATIVE MAP of COTE D'ivoire Map Nº: 01-000-June-2005 COTE D'ivoire 2Nd Edition
ADMINISTRATIVE MAP OF COTE D'IVOIRE Map Nº: 01-000-June-2005 COTE D'IVOIRE 2nd Edition 8°0'0"W 7°0'0"W 6°0'0"W 5°0'0"W 4°0'0"W 3°0'0"W 11°0'0"N 11°0'0"N M A L I Papara Débété ! !. Zanasso ! Diamankani ! TENGRELA [! ± San Koronani Kimbirila-Nord ! Toumoukoro Kanakono ! ! ! ! ! !. Ouelli Lomara Ouamélhoro Bolona ! ! Mahandiana-Sokourani Tienko ! ! B U R K I N A F A S O !. Kouban Bougou ! Blésségué ! Sokoro ! Niéllé Tahara Tiogo !. ! ! Katogo Mahalé ! ! ! Solognougo Ouara Diawala Tienny ! Tiorotiérié ! ! !. Kaouara Sananférédougou ! ! Sanhala Sandrégué Nambingué Goulia ! ! ! 10°0'0"N Tindara Minigan !. ! Kaloa !. ! M'Bengué N'dénou !. ! Ouangolodougou 10°0'0"N !. ! Tounvré Baya Fengolo ! ! Poungbé !. Kouto ! Samantiguila Kaniasso Monogo Nakélé ! ! Mamougoula ! !. !. ! Manadoun Kouroumba !.Gbon !.Kasséré Katiali ! ! ! !. Banankoro ! Landiougou Pitiengomon Doropo Dabadougou-Mafélé !. Kolia ! Tougbo Gogo ! Kimbirila Sud Nambonkaha ! ! ! ! Dembasso ! Tiasso DENGUELE REGION ! Samango ! SAVANES REGION ! ! Danoa Ngoloblasso Fononvogo ! Siansoba Taoura ! SODEFEL Varalé ! Nganon ! ! ! Madiani Niofouin Niofouin Gbéléban !. !. Village A Nyamoin !. Dabadougou Sinémentiali ! FERKESSEDOUGOU Téhini ! ! Koni ! Lafokpokaha !. Angai Tiémé ! ! [! Ouango-Fitini ! Lataha !. Village B ! !. Bodonon ! ! Seydougou ODIENNE BOUNDIALI Ponondougou Nangakaha ! ! Sokoro 1 Kokoun [! ! ! M'bengué-Bougou !. ! Séguétiélé ! Nangoukaha Balékaha /" Siempurgo ! ! Village C !. ! ! Koumbala Lingoho ! Bouko Koumbolokoro Nazinékaha Kounzié ! ! KORHOGO Nongotiénékaha Togoniéré ! Sirana -

Côte D'ivoire
AFRICAN DEVELOPMENT FUND PROJECT COMPLETION REPORT HOSPITAL INFRASTRUCTURE REHABILITATION AND BASIC HEALTHCARE SUPPORT REPUBLIC OF COTE D’IVOIRE COUNTRY DEPARTMENT OCDW WEST REGION MARCH-APRIL 2000 SCCD : N.G. TABLE OF CONTENTS Page CURRENCY EQUIVALENTS, WEIGHTS AND MEASUREMENTS ACRONYMS AND ABBREVIATIONS, LIST OF ANNEXES, SUMMARY, CONCLUSION AND RECOMMENDATIONS BASIC DATA AND PROJECT MATRIX i to xii 1 INTRODUCTION 1 2 PROJECT OBJECTIVES AND DESIGN 1 2.1 Project Objectives 1 2.2 Project Description 2 2.3 Project Design 3 3. PROJECT IMPLEMENTATION 3 3.1 Entry into Force and Start-up 3 3.2 Modifications 3 3.3 Implementation Schedule 5 3.4 Quarterly Reports and Accounts Audit 5 3.5 Procurement of Goods and Services 5 3.6 Costs, Sources of Finance and Disbursements 6 4 PROJECT PERFORMANCE AND RESULTS 7 4.1 Operational Performance 7 4.2 Institutional Performance 9 4.3 Performance of Consultants, Contractors and Suppliers 10 5 SOCIAL AND ENVIRONMENTAL IMPACT 11 5.1 Social Impact 11 5.2 Environmental Impact 12 6. SUSTAINABILITY 12 6.1 Infrastructure 12 6.2 Equipment Maintenance 12 6.3 Cost Recovery 12 6.4 Health Staff 12 7. BANK’S AND BORROWER’S PERFORMANCE 13 7.1 Bank’s Performance 13 7.2 Borrower’s Performance 13 8. OVERALL PERFORMANCE AND RATING 13 9. CONCLUSIONS, LESSONS AND RECOMMENDATIONS 13 9.1 Conclusions 13 9.2 Lessons 14 9.3 Recommendations 14 Mrs. B. BA (Public Health Expert) and a Consulting Architect prepared this report following their project completion mission in the Republic of Cote d’Ivoire on March-April 2000. -

Study of the Biomass Potential in Côte D'ivoire
Study of the biomass potential in Côte d'Ivoire Commissioned by the Netherlands Enterprise Agency Sector study Waste-based Biomass in Côte d’Ivoire RVO project ref. 202009047 / PST20CI02 Study of the biomass potential in Côte d'Ivoire Final Report Marius Guero / Bregje Drion / Peter Karsch | Partners for Innovation | 4 June 2021 Contact persons: Mrs. E. Bindels (RVO) and Mr. J. Kouame (EKN Côte d’Ivoire) Toyola cookstoves – Ghana [photo Toyola] Contents 1. Introduction .............................................................................................................. 1 1.1 Background .......................................................................................................................... 1 1.2 Purpose of the study ........................................................................................................... 1 1.3 Study outline........................................................................................................................ 1 1.4 Guidance for the reader ...................................................................................................... 2 2. Approach of the inventory and selection of productive use cases................................ 3 2.1 Context ................................................................................................................................ 3 2.2 Approach of the inventory .................................................................................................. 3 2.3 Methodology to identify, assess and select the -

Côte D'ivoire
Côte d’Ivoire Risk-sensitive Budget Review UN Office for Disaster Risk Reduction UNDRR Country Reports on Public Investment Planning for Disaster Risk Reduction This series is designed to make available to a wider readership selected studies on public investment planning for disaster risk reduction (DRR) in cooperation with Member States. United Nations Office for Disaster Risk Reduction (UNDRR) Country Reports do not represent the official views of UNDRR or of its member countries. The opinions expressed and arguments employed are those of the author(s). Country Reports describe preliminary results or research in progress by the author(s) and are published to stimulate discussion on a broad range of issues on DRR. Funded by the European Union Front cover photo credit: Anouk Delafortrie, EC/ECHO. ECHO’s aid supports the improvement of food security and social cohesion in areas affected by the conflict. Page i Table of contents List of figures ....................................................................................................................................ii List of tables .....................................................................................................................................iii List of acronyms ...............................................................................................................................iv Acknowledgements ...........................................................................................................................v Executive summary ......................................................................................................................... -

Populated Printable COP 2009 Cote D'ivoire Generated 9/28/2009 12:01:26 AM
Populated Printable COP 2009 Cote d'Ivoire Generated 9/28/2009 12:01:26 AM ***pages: 518*** Cote d'Ivoire Page 1 Table 1: Overview Executive Summary File Name Content Type Date Uploaded Description Uploaded By COP09 Exec Summary- application/msword 11/14/2008 CI Executive Summary OTossou bh-bbs-bh-13nov08.doc Country Program Strategic Overview Will you be submitting changes to your country's 5-Year Strategy this year? If so, please briefly describe the changes you will be submitting. Yes X No Description: Ambassador Letter File Name Content Type Date Uploaded Description Uploaded By Ambassador letter-CI- application/pdf 11/14/2008 CI Ambassador Letter OTossou 14nov08.pdf Country Contacts Contact Type First Name Last Name Title Email PEPFAR Coordinator Brian Howard COP Manager (not Country [email protected] Coordinator) PEPFAR Coordinator James Allman Interim Coordinator [email protected] DOD In-Country Contact Patrick Doyle Defense Attaché [email protected] HHS/CDC In-Country Contact Bruce Struminger CDC Chief of Party [email protected] USAID In-Country Contact Toussaint Sibailly USAID Focal Point [email protected] U.S. Embassy In-Country Cynthia Akuetteh DCM [email protected] Contact Global Fund What is the planned funding for Global Fund Technical Assistance in FY 2009? $350000 Does the USG assist GFATM proposal writing? Yes Does the USG participate on the CCM? Yes Generated 9/28/2009 12:01:26 AM ***pages: 518*** Cote d'Ivoire Page 2 Table 2: Prevention, Care, and Treatment Targets 2.1 Targets for Reporting Period Ending -

Ivory Coast: Administrative Structure
INFORMATION PAPER Ivory Coast – Administrative Structure The administrative structure of Ivory Coast1 was revised in September 2011. The new structure, which consists of 14 districts (2 autonomous districts and 12 regular districts) at first-order (ADM1) level, is as follows: ADM1 – 14 districts (2 autonomous districts and 12 regular districts) ADM2 – 31 regions (fra: région) ADM3 – 95 departments (fra: départment) ADM4 – 498 sub-prefectures (fra: sous-préfecture) Details of the ADM1s and ADM2s are provided on the next page. A map showing the administrative divisions can be found here: http://www.gouv.ci/doc/1333118154nouveau_decoupage_administrative_ci.pdf The previous structure, consisting of 19 regions at first-order level, was reorganised as follows: 1. The cities of Abidjan and Yamoussoukro were split from their regions (Lagunes and Lacs, respectively) to form autonomous districts. 2. The northern regions of Denguélé, Savanes, Vallée du Bandama, and Zanzan were re- designated as districts with no change in territory. 3. The old Agnéby and Lagunes regions, excluding Abidjan (see no. 1), merged to form Lagunes district. 4. Bafing and Worodougou regions merged to form Woroba district. 5. The department of Fresco was transferred from Sud-Bandama to Bas-Sassandra region to form Bas-Sassandra district; the remainder of Sud-Bandama region merged with Fromager to form Gôh-Djiboua district. 6. Dix-Huit Montagnes (18 Montagnes) and Moyen-Cavally regions merged to form Montagnes district. 7. Haut-Sassandra and Marahoué regions merged to form Sassandra-Marahoué district. 8. N'zi-Comoé and Lacs regions, excluding Yamoussoukro (see no. 1), merged to form Lacs district. 9. Moyen-Comoé and Sud-Comoé regions merged to form Comoé district. -

PDF En Anglais
Report No: AUS10013 Republic of Côte d'Ivoire Côte d'Ivoire Urbanization Review June 12, 2015 . GSURR AFRICA i Standard Disclaimer: This volume is a product of the staff of the International Bank for Reconstruction and Development/ The World Bank. The findings, interpretations, and conclusions expressed in this paper do not necessarily reflect the views of the Executive Directors of The World Bank or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. Copyright Statement: The material in this publication is copyrighted. Copying and/or transmitting portions or all of this work without permission may be a violation of applicable law. The International Bank for Reconstruction and Development/ The World Bank encourages dissemination of its work and will normally grant permission to reproduce portions of the work promptly. For permission to photocopy or reprint any part of this work, please send a request with complete information to the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, USA, telephone 978-750-8400, fax 978-750-4470, http://www.copyright.com/. All other queries on rights and licenses, including subsidiary rights, should be addressed to the Office of the Publisher, The World Bank, 1818 H Street NW, Washington, DC 20433, USA, fax 202-522-2422, e-mail [email protected]. -

Côte D'ivoire Country Operational Plan (COP) 2017
Côte d’Ivoire Country Operational Plan (COP) 2017 Strategic Direction Summary March 2017 1 | Page Table of Contents 1.0 Goal Statement 2.0 Epidemic, Response, and Program Context 2.1 Summary statistics, disease burden and epidemic profile 2.2 Investment profile 2.3 National Sustainability Profile 2.4 Stakeholder engagement 3.0 Geographic and Population Prioritization 4.0 Program Activities for Epidemic Control in Scale-up Locations and Populations 4.1 Targets for scale-up locations and populations 4.2 Priority and key population prevention 4.3 Preventing mother-to-child transmission (PMTCT) 4.4 HIV testing services (HTS) 4.5 Facility and community-based care and support 4.6 Tuberculosis and HIV co-infection (TB/HIV) 4.7 Adult treatment 4.8 Pediatric treatment 4.9 Orphans and vulnerable children (OVC) 4.10 Laboratory 4.11 Commodities 4.12 Collaboration, Integration, and Monitoring 5.0 Program Activities for Epidemic Control in Sustained Locations and Populations 5.1 Targets for Sustained locations and populations 5.2 Package of services in sustained support locations and populations 6.0 Program Support Necessary to Achieve Sustained Epidemic Control 6.1 Critical systems investments for achieving key programmatic gaps 6.2 Critical systems investments for achieving priority policies 6.3 Proposed system investments outside of programmatic gaps and priority policies 7.0 USG Management, Operations, and Staffing Plan to Achieve Stated Goals Appendix A – Prioritization Appendix B - Budget Profile and Resource Projections Appendix C - Tables and Systems Investments for Section 6.0 2 | Page 1.0 Goal Statement The President’s Emergency Plan for AIDS Relief (PEPFAR) program in Côte d’Ivoire will contribute to achieving the UNAIDS 90:90:90 goals embraced by the Ivorian government and multilateral stakeholders, by increasing access to quality combination prevention services, improving antiretroviral therapy (ART) coverage, and scaling up Viral Load (VL) testing services. -

Côte D'ivoire Benin OCHA-CI/CNTIG Sierra Leone Togo Cote D'ivoire Ghana Liberia
Côte d’Ivoire Post-Conflict Environmental Assessment United Nations Environment Programme First published in July 2015 by the United Nations Environment Programme. © 2015, United Nations Environment Programme. ISBN: 978-92-807-3461-4 Job No.: DEP/1913/GE United Nations Environment Programme P.O. Box 30552 Nairobi, KENYA Tel: +254 (0)20 762 1234 Fax: +254 (0)20 762 3927 E-mail: [email protected] Web: http://www.unep.org This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder provided acknowledgement of the source is made. UNEP would appreciate receiving a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from UNEP. The designation of geographical entities in this report, and the presentation of the material herein, do not imply the expression of any opinion whatsoever on the part of the publisher or the participating organisations concerning the legal status of any country, territory or area, or of its authorities, or concerning the delimination of its frontiers or boundaries. Unless otherwise credited, all the photographs in this publication were taken by the UNEP assessment team. UNEP promotes Cover Design and Layout: Matija Potocnik Maps and Remote Sensing: Yves Barthélemy environmentally sound practices Cover Image: © Matija Potocnik globally and in its own activities. This publication is printed on recycled paper using vegetable-based inks and other eco- friendly practices. -

Sustainable Management of Nestlé's Cocoa Supply Chain in the Ivory
Improving Workers’ Lives Worldwide Sustainable Management of Nestlé’s Cocoa Supply Chain in the Ivory Coast—Focus on Labor Standards JUNE 2012 Executive Summary In November 2011, FLA commissioned a team of 20 local and international experts to conduct an assessment of Nestlé’s cocoa supply chain in the Ivory Coast. The assessment team included representatives from the Centre de Recherche et d’Action pour la Paix, Abidjan; Afrique Secours et Assistance; Human Resources Without Borders; the Sustainable Livelihoods Foundation, and the FLA. The goals of the assessment were to: map stakeholders in Nestlé’s cocoa supply chain; map Nestlé’s cocoa supply chain in the Ivory Coast; and assess the associated labor risks in Nestlé’s cocoa supply chain. FLA’s assessment of the cocoa supply chain builds on existing research and focuses not on counting the number of children working in the Discussion with farmers in a camp industry, but rather on evaluating the root causes and means available to build a robust monitoring and remediation system. The report identifies gaps in Nestlé’s internal management systems and their effect on labor risks in the supply chain. The report also provides detailed recommendations to Nestlé, the government, and other international buyers on how to mitigate risks to workers throughout the global supply chain. The field visits to the Ivory Coast were initially planned for December 2011, but due to security reasons around parliamentary elections, the visits by the assessment team were delayed until January 2012. To develop a full understanding of the risks facing workers in the sector, the assessment team consulted with a number of government institutions, civil society organizations and local associations in the Ivory Coast. -

Abidjan (Sduga)
Republic of Côte d'Ivoire Ministry of Construction, Housing, Sanitation and Urban Development (MCLAU) REPUBLIC OF COTE D’IVOIRE THE PROJECT FOR THE DEVELOPMENT OF THE URBAN MASTER PLAN IN GREATER ABIDJAN (SDUGA) FINAL REPORT VOLUME III URBAN TRANSPORT MASTER PLAN FOR GREATER ABIDJAN MARCH 2015 JAPAN INTERNATIONAL COOPERATION AGENCY (JICA) Oriental Consultants Global Co., Ltd. Japan Development Institute International Development Center of Japan Asia Air Survey Co., Ltd. Exchange Rate EUR 1.00 = FCFA 655.957 = USD 1.31 = JPY 139.42 September 2014 S CHEMA DIRECTEUR d’ U RBANISME du G RAND A BIDJAN Table of Contents VOLUME III URBAN TRANSPORT MASTER PLAN FOR GREATER ABIDJAN Abbreviations v Part 5 Current Conditions and Planning Prerequisites for urban Transport Master Plan 1.0 Existing Conditions and Salient Findings .................................................. 1 1.1 Road ................................................................................................................ 1 1.2 Public Transport ............................................................................................ 22 1.3 Railway .......................................................................................................... 36 2.0 Transport-Related Surveys ........................................................................ 55 2.1 General ......................................................................................................... 55 2.2 Household Interview Survey .........................................................................