Predicting Suitable Environments and Potential Occurrences for Cinnamomum Camphora (Linn.) Presl
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

October 2004
$WODQWLF5KRGR ZZZ$WODQWLF5KRGRRUJ 9ROXPH1XPEHU 2FWREHU 2FWREHU 3RVLWLRQVRI5HVSRQVLELOLW\ President Penny Gael 826-2440 Director - Social Sandy Brown 683-2615 Vice-President Available Director - R.S.C. Horticulture Audrey Fralic 683-2711 (National) Rep. Sheila Stevenson 479-3740 Director Anitra Laycock 852-2502 Secretary Lyla MacLean 466-449 Newsletter Mary Helleiner 429-0213 Treasurer Chris Hopgood 479-0811 Website Tom Waters 429-3912 Membership Betty MacDonald 852-2779 Library Shirley McIntyre 835-3673 Past President Sheila Stevenson 479-3740 Seed Exchange Sharon Bryson 863-6307 Director - Education Jenny Sandison 624-9013 May - Advance Plant Sale Ken Shannik 422-2413 Director - Communications Mary Helleiner 429-0213 May- Public Plant Sale Duff & Donna Evers 835-2586 0HPEHUVKLS Fees are due on January 1, 2005. Annual dues are $ 15.00 for individuals or families. Make cheques payable to Atlantic Rhododendron and Horticultural Society. Send them to ARHS Membership Secretary, Betty MacDonald, 534 Prospect Bay Road, Prospect Bay, NS B3T1Z8. Please renew your membership now. When renewing, please include your telephone number and e-mail. This information will be used for Society purposes only (co-ordination of potluck suppers and other events) and will be kept strictly confidential. The Website address for the American Rhododendron Society is www.rhododendron.org for those wishing to renew their membership or become new members of the ARS. AtlanticRhodo is the Newsletter of the Atlantic Rhododendron and Horticultural Society. We welcome your comments, suggestions, articles, photos and other material for publication. Send all material to the editor. (GLWRU 0DU\ +HOOHLQHU 0DUOERURXJK $YH Published three times a year. February, May and October. -

Sustainable Sourcing : Markets for Certified Chinese
SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS In collaboration with SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS Abstract for trade information services ID=43163 2016 SITC-292.4 SUS International Trade Centre (ITC) Sustainable Sourcing: Markets for Certified Chinese Medicinal and Aromatic Plants. Geneva: ITC, 2016. xvi, 141 pages (Technical paper) Doc. No. SC-2016-5.E This study on the market potential of sustainably wild-collected botanical ingredients originating from the People’s Republic of China with fair and organic certifications provides an overview of current export trade in both wild-collected and cultivated botanical, algal and fungal ingredients from China, market segments such as the fair trade and organic sectors, and the market trends for certified ingredients. It also investigates which international standards would be the most appropriate and applicable to the special case of China in consideration of its biodiversity conservation efforts in traditional wild collection communities and regions, and includes bibliographical references (pp. 139–140). Descriptors: Medicinal Plants, Spices, Certification, Organic Products, Fair Trade, China, Market Research English For further information on this technical paper, contact Mr. Alexander Kasterine ([email protected]) The International Trade Centre (ITC) is the joint agency of the World Trade Organization and the United Nations. ITC, Palais des Nations, 1211 Geneva 10, Switzerland (www.intracen.org) Suggested citation: International Trade Centre (2016). Sustainable Sourcing: Markets for Certified Chinese Medicinal and Aromatic Plants, International Trade Centre, Geneva, Switzerland. This publication has been produced with the financial assistance of the European Union. -

Documentation of Reticulate Evolution in Peonies (Paeonia) Using
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 6813-6817, July 1995 Evolution Documentation of reticulate evolution in peonies (Paeonia) using internal transcribed spacer sequences of nuclear ribosomal DNA: Implications for biogeography and concerted evolution TAO SANG*, DANIEL J. CRAWFORD, AND TOD F. STUESSY Department of Plant Biology, Ohio State University, Columbus, OH 43210-1293 Communicated by Michael T. Clegg, University of California, Riverside, CA, April 17, 1995 ABSTRACT The internal transcribed spacers (ITS) of genus, comprising three sections and -35 diploid (2n = 10) nuclear ribosomal DNA of 33 species of genus Paeonui (Paeo- and tetraploid species of shrubs and perennial herbs, occurs niaceae) were sequenced. In section Paeonia, different pat- widely in disjunct areas of the northern temperate region terns of nucleotide additivity were detected in 14 diploid and (11-13). All the tetraploid species belong to herbaceous sec- tetraploid species at sites that are variable in the other 12 tion Paeonia. The majority of the tetraploids occurring in the species of the section, suggesting that reticulate evolution has Mediterranean region has been suggested as allopolyploids (9, occurred. Phylogenetic relationships of species that do not 10). The origins of the putative allotetraploids, however, show additivity, and thus ostensibly were not derived through remain unknown. The diploid species ofthis section have never hybridization, were reconstructed by parsimony analysis. The been considered to be of hybrid origin. taxa presumably derived through reticulate evolution were In the present study, ITS sequences were used to reconstruct then added to the phylogenetic tree according to additivity the phylogeny of Paeonia. t The sequence data indicate that from putative parents. -

Pollen Morphology of Herbaceous Peonies with Different Ploidy Levels
J. AMER.SOC.HORT.SCI. 141(3):275–284. 2016. Pollen Morphology of Herbaceous Peonies with Different Ploidy Levels Lihong Hao and Hui Ma College of Landscape Architecture, Beijing Forestry University, Beijing, 100083, P.R. China Jaime A. Teixeira da Silva P.O. Box 7, Miki-cho post office, Ikenobe 3011-2, Kagawa-ken, 761-0799, Japan XiaoNan Yu1 College of Landscape Architecture, Beijing Forestry University, Beijing, 100083, P.R. China; and Beijing Key Laboratory of Ornamental Plants Germplasm Innovation and Molecular Breeding, National Engineering Research Center for Floriculture, Beijing, 100083, P.R. China ADDITIONAL INDEX WORDS. Paeonia ABSTRACT. The pollen morphology of 26 cultivars of herbaceous peony (Paeonia L.) was examined by scanning electron microscopy (SEM). Pollen grains of the cultivars were mostly subprolate to perprolate, tricolporate, or ellipsoidal in equatorial view, but rounded-trifid in polar view. The poles of pollen grains were mainly flat when viewed in cross-section and only a few appeared to have a circular or arc shape. In addition, the surface of the pollen grains was psilate, and the muri were partly or fully protuberant with a reticulate surface sculpture. However, the shapes and sizes of pollen grains as well as surface ornamentation varied significantly among cultivars with different chromosome numbers. The pollen grains from diploid cultivars were regular and full, and the reticulation holes were evenly distributed, while most of the pollen grains from triploid cultivars were empty flat shells with some heteromorphic pollen whose surface ornamentation was mostly rugulate-reticulate exine. The pollen grains from tetraploid cultivars were the largest although pollen morphology and surface ornamentation differed between Athena and Cream Delight. -

King of Flowers: Reinterpretation of Chinese Peonies in Early Modern Europe
Journal of the Southern Association for the History of Medicine and Science Volume 3 (no. 1) 2021 https://journals.troy.edu/index.php/JSAHMS/ King of Flowers: Reinterpretation of Chinese Peonies in Early Modern Europe Richard Zhang, MA MD Student, Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, Pennsylvania, United States Email: [email protected] Abstract This work argues that the introduction of Chinese peony variants into early modern Europe not only incorporated them into a new, systematic, and universalizing taxonomic body of knowledge, but also accompanied a narrowed translation of their uses that exemplified agnotology, or culturally-induced ignorance. Cultivated in China at least since the Tang Dynasty, both herbaceous and “tree” peonies traditionally enjoyed important medicinal applications and symbolic purposes there, in addition to serving as ornamental garden flowers. Yet, their introduction into Europe beginning in the late eighteenth century by naturalists such as Sir Joseph Banks saw their use confined, albeit popularly, to the latter ornamental use. This research draws upon classical bencao texts of Chinese medicine, early modern correspondence, and printed books to capture how different cultures may utilize and construe the same material objects in markedly contrasting ways. Additionally, quotes from early modern physicians such as Menuret de Chambaud and John Floyer help illustrate European confusion and disregard for concepts from the Chinese worldview such as qi, which likely contributed to medicinal understandings of Chinese peonies not traveling with the actual plants themselves into Europe. This work finally references lately-emerging pharmacologic literature on peonies to support biomedical inquiry into traditional medical materials worldwide, for the potential benefit of broader patient populations. -

PEONIES: Comparative Study by Anatomy and TLC of Three Traditional Chinese Medicinal Plants
Chinese Medicine, 2013, 4, 166-172 Published Online December 2013 (http://www.scirp.org/journal/cm) http://dx.doi.org/10.4236/cm.2013.44020 PEONIES: Comparative Study by Anatomy and TLC of Three Traditional Chinese Medicinal Plants F. El Babili1*, M. El Babili2, I. Fouraste3, C. Chatelain1 1Faculté des Sciences Pharmaceutiques, Laboratoire de BOTANIQUE, Toulouse, France 2Université Claude Bernard, Lyon I, Institut Michel Pacha, La Seyne sur Mer, France 3Faculté des Sciences Pharmaceutiques, Laboratoire de Pharmacognosie, Toulouse, France Email: *[email protected] Received September 23, 2013; revised November 2, 2013; accepted November 26, 2013 Copyright © 2013 F. El Babili et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Anatomical and TLC study of three Chinese peonies were conducted to make a comparative analysis. Peonies (Paeonia suffruticosa (tree peony), Paeonia lactiflora (Chinese peony) and Paeonia veitchii (Chinese peony)) are traditionally used on the Qinghai-Tibet Plateau in China. Recent studies have shown that the peonies have different pharmacological activities and clinical applications. To distinguish these three species of peonies and ensure the safety and effectiveness in their use, the microscopic characteristics and chromatographic profile of their roots and the corresponding powder were studied. Plant materials sectioned and stained and the raw powder were studied with an optical microscope using standard techniques in microscopy. The results of microscopic features and TLC were described and illustrated. The three species have different microscopic characteristics and TLC profiles, which allow us to distinguish them. -
![UNITED STATES DEPARTMENT of , Ai Rionlti] R](https://docslib.b-cdn.net/cover/4564/united-states-department-of-ai-rionlti-r-3324564.webp)
UNITED STATES DEPARTMENT of , Ai Rionlti] R
L Ib H A H T RECEIVED MAR 1 19' UNITED STATES DEPARTMENT OF , Ai rionlti] r INVENTORY No. 87 Washington, D. C. T Issued February, 1929 PLANT MATERIAL INTRODUCED BY THE OFFICE OF FOREIGN PUNT INTRODUCTION, BUREAU OF PLANT INDUSTRY, APRIL 1 TO JUNE 30, 1926 (NOS. 66699 TO 67836) CONTENTS Pag* Introductory statement - 1< Inventory - 3 Index of common and scientific names— .-._. „. ,. — 49 INTRODUCTORY STATEMENT agricultural explorers were carrying on their investigations in foreign lands during the three-month period represented by this eighty-seventh inventory. David Fairchild, in company with P. H. Dorsett, made an extended tour along the northern coast of Sumatra and also spent some time in Java and Ceylon. Their itinerary included the Sibolangit Botanic Garden, near Medan, Sumatra, and the Hakgala Botanic Garden, Newara Eliya, Ceylon. The material collected came from these botanic gardens, from the markets of the native villages visited, and from the wild. It consisted for the most part of fruit-bearing plants, ornamentals, and leguminous plants of possible value as cover crops for the warmer parts of the United States. Breeders of small fruits will be interested in the numerous species of Rubus (Nos. 67592 to 67604; 67728 to 67740) obtained mostly in Sumatra. Sev- eral species of Ficus (Nos. 67557 to 67570; 67696 to 67705) from Sumatra will be tested in southern Florida, where already a number of these wild figs have proved popular as shade trees. F. A. McClure continued to work in the general vicinity of Can- ton, China, collecting plant material largely from the native markets of the neighboring villages. -
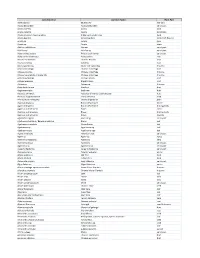
Testing Services List V2020
Latin Binomial Common Name Plant Part Abies sibirica Siberian Fir leaf (oil) Acacia Berlandieri Acacia Berlandieri aerial part Acacia catechu Acacia bark Acacia catechu Acacia gum/resin Acacia nilotica / Acacia arabica Indian gum arabic tree bark Acacia Rigidula Acacia Rigidula herb (leaf, flower) Acacia sp. Acacia gum Acacia sp. Acacia stem Achillea millefolium Yarrow aerial part Achillea sp. Achillea sp. aerial part Achyranthes aspera Prickly chaff flower aerial part Achyranthes bidentata Achyranthes root Aconite carmichaeli Chinese Aconite root Acorus calamus Calamus root Acorus gramineus Grass-leaf sweetflag rhizome Actaea cimicifuga Chinese cimicifuga root Actaea dahurica Chinese cimicifuga rhizome Actaea heracleifolia / Sheng Ma Chinese cimicifuga rhizome Actaea podocarpa Yellow Cohosh root Actaea racemosa Black Cohosh root Actaea sp. Actaea sp. rhizome Actinidia deliciosa Kiwifruit fruit Aegle marmelos Bael tree fruit Aesculus chinensis Aesculus chinensis [Sapindaceae] fruit Aesculus hippocastanum Horse chestnut seed Aframomum melegueta Grains of paradise grain Agaricus bisporus Button Mushroom entire Agaricus bisporus Button Mushroom fruiting body Agaricus subrufescens Blazei entire Agaricus subrufescens Blazei fruiting body Agaricus subrufescens Blazei mycelia Agastache rugosa Huo Xiang aerial part Agathosma betulina / Barosma betulina Buchu leaf Agathosma crenulata Ovate Buchu leaf Agathosma sp. Agathosma sp. leaf Agathosma spp. Agathosma spp. leaf Agave americana American aloe aerial part Agave sp. Agave sp. syrup Agrimonia -

Predicting the Potential Distribution of Paeonia Veitchii (Paeoniaceae) in China by Incorporating Climate Change Into a Maxent Model
Article Predicting the Potential Distribution of Paeonia veitchii (Paeoniaceae) in China by Incorporating Climate Change into a Maxent Model Keliang Zhang, Yin Zhang and Jun Tao * Jiangsu Key Laboratory of Crop Genetics and Physiology, College of Horticulture and Plant Protection, Yangzhou University, Yangzhou 225009, China; [email protected] (K.Z.); [email protected] (Y.Z.) * Correspondence: [email protected]; Tel.: +86-0514-8799-7219 Received: 14 January 2019; Accepted: 19 February 2019; Published: 20 February 2019 Abstract: A detailed understanding of species distribution is usually a prerequisite for the rehabilitation and utilization of species in an ecosystem. Paeonia veitchii (Paeoniaceae), which is an endemic species of China, is an ornamental and medicinal plant that features high economic and ecological values. With the decrease of its population in recent decades, it has become a locally endangered species. In present study, we modeled the potential distribution of P. veitchii under current and future conditions, and evaluated the importance of the factors that shape its distribution. The results revealed a highly and moderately suitable habitat for P. veitchii that encompassed ca. 605,114 km2. The central area lies in northwest Sichuan Province. Elevation, temperature seasonality, annual mean precipitation, and precipitation seasonality were identified as the most important factors shaping the distribution of P. veitchii. Under the scenario with a low concentration of greenhouse gas emissions (RCP 2.6), we predicted an overall expansion of the potential distribution by 2050, followed by a slight contraction in 2070. However, with the scenario featuring intense greenhouse gas emissions (RCP 8.5), the range of suitable habitat should increase with the increasing intensity of global warming. -

Complete Chloroplast Genome Sequence and Phylogenetic Analysis of Paeonia Ostii
molecules Article Complete Chloroplast Genome Sequence and Phylogenetic Analysis of Paeonia ostii Shuai Guo 1,2,†, Lili Guo 1,†, Wei Zhao 1,†, Jiang Xu 2, Yuying Li 1, Xiaoyan Zhang 2,3, Xiaofeng Shen 2, Mingli Wu 2,4 and Xiaogai Hou 1,* 1 College of Agricultural (College of Tree Peony), Henan University of Science and Technology, Luoyang 471023, Henan, China; [email protected] (S.G.); [email protected] (L.G.); [email protected] (W.Z.); [email protected] (Y.L.) 2 Institute of Chinese Materia Medical, China Academy of Chinese Medical Sciences, Beijing 100700, China; [email protected] (J.X.); [email protected] (X.Z); [email protected] (X.S.); [email protected] (M.W.) 3 College of Life Science, Huaibei Normal University, Huaibei 235000, Anhui, China 4 College of Pharmacy, Hubei University of Chinese Medicine, Wuhan 430065, Hubei China * Correspondence: [email protected]; Tel: +86-0379-6998-0776 † These authors contributed equally to this work. Received: 22 December 2017; Accepted: 24 January 2018; Published: 26 January 2018 Abstract: Paeonia ostii, a common oil-tree peony, is important ornamentally and medicinally. However, there are few studies on the chloroplast genome of Paeonia ostii. We sequenced and analyzed the complete chloroplast genome of P. ostii. The size of the P. ostii chloroplast genome is 152,153 bp, including a large single-copy region (85,373 bp), a small single-copy region (17,054 bp), and a pair of inverted repeats regions (24,863 bp). The P. ostii chloroplast genome encodes 111 genes, including 77 protein-coding genes, four ribosomal RNA genes, and 30 transfer RNA genes. -

Next-Generation Genome Sequencing of Sedum Plumbizincicola Sheds
plants Article Next-Generation Genome Sequencing of Sedum plumbizincicola Sheds Light on the Structural Evolution of Plastid rRNA Operon and Phylogenetic Implications within Saxifragales 1,2, 1, 1 3 1 1 Hengwu Ding y, Ran Zhu y, Jinxiu Dong , De Bi , Lan Jiang , Juhua Zeng , Qingyu Huang 1, Huan Liu 3, Wenzhong Xu 4, Longhua Wu 3,* and Xianzhao Kan 1,2,* 1 The Institute of Bioinformatics, College of Life Sciences, Anhui Normal University, Wuhu 241000, Anhui, China; [email protected] (H.D.); [email protected] (R.Z.); [email protected] (J.D.); [email protected] (L.J.); [email protected] (J.Z.); [email protected] (Q.H.) 2 The Provincial Key Laboratory of the Conservation and Exploitation Research of Biological Resources in Anhui, Wuhu 241000, Anhui, China 3 National Engineering Laboratory of Soil Pollution Control and Remediation Technologies, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, Jiangsu, China; [email protected] (D.B.); [email protected] (H.L.) 4 Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; [email protected] * Correspondence: [email protected] (L.W.); [email protected] (X.K.); Tel.: +86-25-8688-1128 (L.W.); +86-139-5537-2268 (X.K.) These authors equally contributed to this work. y Received: 27 August 2019; Accepted: 28 September 2019; Published: 29 September 2019 Abstract: The genus Sedum, with about 470 recognized species, is classified in the family Crassulaceae of the order Saxifragales. Phylogenetic relationships within the Saxifragales are still unresolved and controversial. -

Reproductive Characteristics As Drivers of Alien Plant Naturalization and Invasion
Reproductive characteristics as drivers of alien plant naturalization and invasion Dissertation submitted for the degree of Doctor of Natural Sciences presented by Mialy Harindra Razanajatovo at the Faculty of Sciences Department of Biology Date of the oral examination: 12 February 2016 First referee: Prof. Dr. Mark van Kleunen Second referee: Prof. Dr. Markus Fischer Konstanzer Online-Publikations-System (KOPS) URL: http://nbn-resolving.de/urn:nbn:de:bsz:352-0-324483 Summary Due to human activity and global movements, many plant species have been introduced to non-native regions where they experience novel abiotic and biotic conditions. Some of these alien species manage to establish reproducing naturalized populations, and some naturalized alien species subsequently become invasive. Invasion by alien plant species can negatively affect native communities and ecosystems, but what gives the alien species an advantage under novel conditions is still not clear. Therefore, identifying the drivers of invasions has become a major goal in invasion ecology. Reproduction is crucial in plant invasions, because propagule supply is required for founding new populations, population maintenance and spread in non-native regions. Baker’s Law, referring to the superior advantage of species capable of uniparental reproduction in establishing after long distance dispersal, has received major interest in explaining plant invasions. However, previous findings regarding Baker’s Law are contradicting. Moreover, there has been an increasing interest in understanding the integration of alien plant species into native plant-pollinator networks but few studies have looked at the pollination ecology of successful (naturalized and invasive) and unsuccessful (non-naturalized and non-invasive) alien plant species.