Green Tea Seed Oil Suppressed Aβ1–42-Induced Behavioral and Cognitive Deficit Via the Aβ-Related Akt Pathway
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tea Seed Oil and Health Properties Fatih Seyis1, Emine
Tea Seed Oil and Health Properties Fatih Seyis1, Emine Yurteri1, Aysel Özcan1 1Recep Tayyip Erdoğan University: Faculty of Agriculture and Natural Science, Field Crops Department, Rize/Turkey, e-mail: [email protected] Abstract: Tea Oil has a mild fragrant flavor that goes with anything. It’s not a heavy oil like Olive Oil, but thinner – more like almond oil. If the taste or “oiliness” of olive oil overpowers your food. Along with its mild taste and pleasant tea-like aroma, this oil touts impressive health benefits. Tea seed oil has a high smoke point, contains more monounsaturated fatty acids than olive oil, contains fewer saturated fatty acids than olive oil, contains high levels of Vitamin E, polyphenol antioxidants and both Omegas 3 and 6, but has less Omega 6 and Polyunsaturated Fats than olive oil. Health Benefits of tea seed oil are: it can be applied topically and consumed internally to obtain its health benefits, camellia oil can be used for skin, hair, has anti-cancer effects, effects boost immunity and reduces oxidative stress. Camellia oil is used for a variety of other purposes, for example for cooking, as machinery lubricant, as ingredient in beauty products like night creams, salves, in hair care products and perfumes and is used to coat iron products to prevent rusting. Key words: Tea, seed oil, health 1. Introduction Like other genera of Camellia (from Theaceae family), the tea plant (C.sinensis) produces large oily seeds. In some countries where tea seed oil is abundantly available, it has been accepted as edible oil (Sahari et al., 2004). -

Extraction and Analysis of Tea (Camellia Sinensis) Seed Oil from Different Clones in Kenya
African Journal of Biotechnology Vol. 12(8), pp. 841-846, 20 February, 2013 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB12.2738 ISSN 1684–5315 ©2013 Academic Journals Full Length Paper Extraction and analysis of tea (Camellia sinensis) seed oil from different clones in Kenya Kelvin Omondi George1,2, Thomas Kinyanjui2*, John Wanyoko3, Okong’o Kelvin Moseti3 and Francis Wachira3 1Bidco Oil Refineries Limited, P. O. Box 7029 Nakuru, Kenya. 2Chemistry Department, Egerton University, P. O. Box 536 Njoro, Nakuru, Kenya. 3Tea Research Foundation of Kenya, P. O. Box 820-20200, Kericho, Kenya. Accepted 22 November, 2012 Kenyan tea (Camellia sinensis) is widely grown for its leaves and is commercialized as black tea. Product diversification and value addition is currently an area of great interest. This study provides data on the physico-chemical properties of Kenyan tea seed oil from selected clones of tea seeds to ascertain its potential applications. Soxhlet extraction using hexane was employed to obtain tea seed oil followed by chemical analysis to assess its properties. Oil yield, iodine value, saponification value, peroxide value, free fatty acids, total polyphenols and antioxidant activity were determined. The oil yields ranged between 16 to 25% w/w. Iodine value was in the range of 86 to 91 g I2/100 g, peroxide value < 3.5 meq O2/kg, saponification value between 182 to 187 mg KOH/g, free fatty acid < 1.5% oleic acid, total polyphenols 0.036 to 0.043 mg/L gallic acid and antioxidant activity of between 14 to 21% 2,2- diphenyl-1-picrylhydrazyl (DPPH) scavenging activity. -

The Worm Turns: Earthworm Cast Reduction on Golf Courses
research research thrive under the conditions required to maintain mid-1990s were applied for grub control but were healthythrive under turfgrass the conditionsand are so required adaptable to maintainthat cul- alsomid-1990s acutely were toxic applied to earthworms for grub (14).control Most but of were the turalhealthy manipulations turfgrass and alone are soare adaptableunlikely tothat resolve cul- olderalso acutely worm-toxic toxic topesticides earthworms can no (14). longer Most be of used the castingtural manipulations problems. Physical alone are removal unlikely of tocasts resolve by onolder turf, worm-toxic and presently pesticides no pesticides can no longerare labeled be used for brushing,casting problems. switching Physical or dragging removal is laborious of casts and by earthwormon turf, and control presently in theno pesticidesUnited States. are labeled for Theofbrushing, only temporary switching worm benefit or dragging (8). is laborious turns: and earthworm control in the United States. of only temporary benefit (8). Peter Lees’ invention Chemical control PeterAn approach Lees’ inventionwidely used used for earthworm earthwormChemicalDuring control the past 20 years the problem of exces-castandAn cast approach suppression reduction widely from used the used early for 20th earthworm century siveDuring earthworm the past castings 20 years interfering the problem with of play exces- on untiland cast about suppression 1960 involved from the the early use 20thof chemical century ongolfsive earthwormcourses, golf sport castings -

Flex Foods & Beverages
• 10-DAY WEIGHT LOSS FLEX FOODS & • 10-DAY CLEANSING BEVERAGES • 10-DAY ATHLETIC WHAT IS A FLEX FOOD? WHAT IS A FLEX BEVERAGE? A Flex Food is a fruit or vegetable that is permitted A Flex Beverage is a vegan liquid permitted on the 10-Day on any 10-Day Transformation. Only 3 servings of Transformation. You can mix one with your Power Shake, Flex Foods OR Beverages are allowed per day, on a MVP Sport, Apothe-Cherry or have one separately. They Transformation. count as one of the three Flex Foods / Beverages you are Flex Foods and Flex Beverages are a way to control allowed per day. your intake, retrain your hunger cravings and fuel your Ideal Flex Beverages body with optimal nutrients. If you stick to your goals, • Hemp, almond, coconut, or oat milk Flex Foods and Flex Beverages will naturally become your go-to snacks, long after your first 10 days. • Kombucha • Organic vegetable broth Ideal Flex Foods • Decaffeinated herbal tea • 1 Avocado • Coconut water • 1 Apple (Tip: Eating apples first thing in the morning can help wake you up.) • Purium green drinks: Organic Kamut Blend, Organic Green Spectrum, Organic Barley Green Juice, Organic • 1 cup Watermelon Spirulina, Chlorella, Power of 10 Veggie • Unlimited Celery Easy Flex Food combinations • Unlimited Cucumbers • A green apple sautéed in cinnamon and Organic Tropic Oil • 1 cup Broccoli, Cauliflower, Kale, or Spinach • An avocado mashed with freshly squeezed lemon juice and • 1 cup Berries Himalayan sea salt – eaten with cucumber “chips” • 1 cup Sauerkraut or Kimchi (no additives) • 1 cup broccoli sautéed in Organic Tropic Oil and fresh basil • 1 cup Summer squash (winter squash not permitted) and topped with 3 tbsp. -

Chemical Composition, Antimicrobial and Antioxidant Properties of Seed Oil Plants of North-East India: a Review
Review Chemical composition, antimicrobial and antioxidant properties of seed oil plants of North-East India: A review Priyanka Saha1, Anupam Das Talukdar1*, Sanjoy Singh Ningthoujam1,2, Manabendra Dutta Choudhury1, Deepa Nath1,3, Lutfun Nahar4, Satyajit Dey Sarker4, Norazah Basar4,5 1Department of Life Science and Bioinformatics, Assam University, Silchar 788011, India; 2Department of Botany, Ghanapriya Women’s College, Imphal, Manipur, India; 3Department of Botany and Biotechnology, Karimganj College, Karimganj-788710. Assam India; 4Medicinal Chemistry and Natural Products Research Group, School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Byrom Street, Liverpool L3 3AF, UK; 5Department of Chemistry, Faculty of Science, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia ABSTRACT Apart from being used as food, seed oils have also been used traditionally as medicinal products by several communities. However, the full medicinal potential of many seed oil plants is yet to be properly reviewed, particularly for their antimicrobial and antioxidant properties. North-East India has rich resources of seed oil plants. The availability of detailed information on these plants is quite limited. This review aims to explore and evaluate these seed oil plants of the North-East India with particular emphasis on their antimicrobial and antioxidant activities as well as chemical compositions. A comprehensive literature search on seed oil plants of this region has been performed. Seed oil yielding plants of this region can be categorized into two categories: plants that are used traditionally as sources of edible or medicinal oils and plants that are used for purposes other than as sources of oils. Many seed oil plants of this region have been reported to possess antimicrobial and antioxidant properties, and to produce various types of compounds. -

A Art of Essential Oils
The Essence’s of Perfume Materials Glen O. Brechbill FRAGRANCE BOOKS INC. www.perfumerbook.com New Jersey - USA 2009 Fragrance Books Inc. @www.perfumerbook.com GLEN O. BRECHBILL “To my parents & brothers family whose faith in my work & abilities made this manuscript possible” II THE ESSENCES OF PERFUME MATERIALS © This book is a work of non-fiction. No part of the book may be used or reproduced in any manner whatsoever without written permission from the author except in the case of brief quotations embodied in critical articles and reviews. Please note the enclosed book is based on The Art of Fragrance Ingredients ©. Designed by Glen O. Brechbill Library of Congress Brechbill, Glen O. The Essence’s of Perfume Materials / Glen O. Brechbill P. cm. 477 pgs. 1. Fragrance Ingredients Non Fiction. 2. Written odor descriptions to facillitate the understanding of the olfactory language. 1. Essential Oils. 2. Aromas. 3. Chemicals. 4. Classification. 5. Source. 6. Art. 7. Thousand’s of fragrances. 8. Science. 9. Creativity. I. Title. Certificate Registry # 1 - 164126868 Copyright © 2009 by Glen O. Brechbill All Rights Reserved PRINTED IN THE UNITED STATES OF AMERICA 10 9 8 7 6 5 4 3 2 1 First Edition Fragrance Books Inc. @www.perfumerbook.com THE ESSENCE’S OF PERFUME MATERIALS III My book displays the very best of essential oils. It offers a rich palette of natural ingredients and essences. At its fullest it expresses a passion for the art of perfume. With one hundred seventy-seven listings it condenses a great deal of pertinent information in a single text. -

Enhancement of Thermal Stability of Soybean Oil by Blending with Tea Seed Oil
Emirates Journal of Food and Agriculture. 2018. 30(11): 968-977 doi: 10.9755/ejfa.2018.v30.i11.1862 http://www.ejfa.me/ REGULAR ARTICLE Enhancement of thermal stability of soybean oil by blending with tea seed oil Nopparat Prabsangob1* and Soottawat Benjakul2 1Department of Product Development, Faculty of Agro-Industry, Kasetsart University, Bangkok 10900 Thailand, 2Department of Food Technology, Faculty of Agro-Industry, Prince of Songkla University, Hat Yai, Songkhla 90112 Thailand ABSTRACT Frying accelerates oil deterioration through several chemical reactions, particularly lipid oxidation. Soybean oil (SBO), the polyunsaturated fatty acid (PUFA) rich oil, is prone to thermal degradation. Nevertheless, tea seed oil (TSO), mainly consisting of monounsaturated fatty acids (MUFA), is quite stable. This work aimed to elucidate thermal stability of SBO as affected by TSO blending at varying volume ratios. After frying for several repeated cycles, SBO/TSO blends with the ratios of 70:30, 60:40 and 50:50 showed lower total oxidative degree than SBO alone. FTIR spectra suggested less cis C=C deformation of the SBO blended with TSO, and the 60:40 SBO/TSO blend contained the lowest secondary oxidation products. Along frying, less change in viscosity (color) was found for the 60:40 and 50:50 (60:40) SBO/TSO blends. Improved thermal stability of the blended oils was expected due to the decrease in PUFA and increase in phenolic content, and this study suggested that the 60:40 SBO/TSO blend showed the highest stability. Keywords: Deep-frying; Oil blending; Soybean oil; Tea seed oil; Thermal stability; Oxidation INTRODUCTION of polyunsaturated fatty acids (PUFA), particularly linoleic acid (Su and White 2004). -
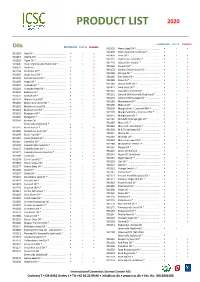
Product List 2020
PRODUCT LIST 2020 CONVENTIONAL ORGANIC STABILIZED Oils CONVENTIONAL ORGANIC STABILIZED 901199 Hemp Seed Oil * .................................. • • • 901499 Hemp Seed Oil Unrefined * ................. • • 901193 Acai Oil * ............................................. • • • 901450 Inchi Oil *............................................. • • • 901367 Alfalfa Oil* ........................................... • • • 901112 Jojoba Oil – Colorless * ........................ • • • 901228 Algae Oil * ........................................... • • • 901110 Jojoba Oil – Golden * ........................... • • • 907440 Aloe Oil (Internally Stabilized)* .......... • • 901162 Kakadu Oil * ........................................ • • • 906221 Amla Oil .............................................. • • 901152 Kalahari Melon Seed Oil * ................... • • • 901148 Andiroba Oil * ..................................... • • • 901168 Karanja Oil * ........................................ • • • 901387 Apple Seed Oil * .................................. • • • 901165 Kiwi Seed Oil * ..................................... • • • 901176 Apricot Kernel Oil * ............................. • • • 901185 Kukui Oil * ........................................... • • • 901195 Argan Oil * ........................................... • • • 901180 Lemon Seed Oil * ................................ • • • 901118 Avocado Oil * ...................................... • • • 901421 Lime Seed Oil * .................................... • • • 901218 Avocado Seed Oil * ............................. -

Camellia As an Oilseed Crop
HORTSCIENCE 52(4):488–497. 2017. doi: 10.21273/HORTSCI11570-16 Camellia as an Oilseed Crop Haiying Liang1 Department of Genetics and Biochemistry, Clemson University, Clemson, SC 29634 Bing-Qing Hao, Guo-Chen Chen, Hang Ye, and Jinlin Ma1 Guangxi Forestry Research Institute, Guangxi Key Laboratory of Non-wood Cash Crops Cultivation and Utilization, Nanning, P.R. China, 530002 Additional index words. biodiesel, cultivar, edible oil, new horticultural crop, oil camellias Abstract. Camellia is one of the four main oil-bearing trees along with olive, palm, and coconut in the world. Known as ‘‘Eastern Olive Oil,’’ camellia oil shares similar chemical composition with olive oil, with high amounts of oleic acid and linoleic acid and low saturated fats. Camellia was first exploited for edible oil in China more than 1000 years ago. Today, its oil serves as the main cooking oil in China’s southern provinces. Introduction of camellia oil into the Western countries was delayed until the recognition of its many health benefits. Although popularity for the oil has yet to grow outside of China, interest has emerged in commercial production of camellia oil in other countries in recent years. Unlike seed-oil plants that are grown on arable land, oil camellias normally grow on mountain slopes. This allows the new crop to take full usage of the marginal lands. To facilitate promoting this valuable crop as an alternative oil source and selecting promising cultivars for targeted habitats, this paper reviews the resources of oil camellias developed in China, use of by-products from oil-refining process, as well as the progress of developing camellias for oil production in China and other nations. -

Review Tea Seed Oil: Extraction, Compositions, Applications
Academia Journal of Medicinal Plants 1(4): 068-079, April 2013 DOI: http://dx.doi.org/10.15413/ajmp.2012.0113 ISSN: 2315-7720 ©2013 Academia Publishing Review Tea seed oil: Extraction, compositions, applications, functional and antioxidant properties Accepted 5th February, 2013 ABSTRACT The beverage tea plant is mainly cultivated for its prolific vegetative growth. Tea plant produced large amounts of oil (30-32%) moreover; its seeds act as functional product with various applications. This oil is one of the important vegetable oils because of the high unsaturated fatty acids, especially essential linoleic acid and low content of saturated fat. The oil of tea seed also lower blood pressure and cholesterol level, and has functional effects against several degenerative pathologies, including cardiovascular diseases and cancers. Tea seed Mohammad Ali Sahari* and Mojtaba oil is a high quality cooking oil, like olive oil; it has an excellent storage quality due Amooi to a high content of polyphenols as antioxidant agents. Tea seed oil also is a good Department of Food Technology, raw material for producing cocoa butter equivalent and margarine. Several studies Faculty of Agriculture, Tarbiat have been developed on tea seed oil extraction, compositions, technical properties, Modares University, Tehran, Iran. and its application in food products which will be reviewed in this article. *Corresponding author. E-mail: [email protected]. Tel: +98-21- Key words: Tea seed oil, Oil extraction, Compositions, Applications, Functional and 48292328. Fax: +98-21-48292200 antioxidant properties INTRODUCTION In some countries where Camellia tea seed oil is abundantly applied as a medicine for stomach ache and burning available, it has been accepted as edible oil (Fazel et al., injuries in China. -

Biochemical Assessment of Possible Protective Role of Kombucha Tea Against Stressful Effect Induced by High Sucrose Dose
JOURNAL OF NATURAL REMEDIES DOI: 10.18311/jnr/2019/23124 Biochemical Assessment of Possible Protective Role of Kombucha Tea against Stressful Effect Induced by High Sucrose Dose H. A. Abdel Maksoud1, Raafat R. Mohammed1, Mohamed G. Elharrif2 and Nedal S. Abdulatif1 1Department of Biochemistry, Benha University, Egypt 2Department of Basic Medical Sciences, Shaqra University, KSA Abstract Kombucha tea is highly fermented beverage popularly consumed in many countries. The aim was to evaluate the possible protective effects of the usage of Kombucha as natural agent against the stressful effects resulted from administration of high sucrose diet to male rabbits through determination of some biochemical parameters. The results demonstrated that pretreatment with Kombucha tea in high sucrose stressed rabbit significantly improve lipid profile and antioxidant system meanwhile significant reduction of glucose, urea, creatinine, cupper and non significant change in testosterone and copper levels. In conclusion, Kombucha tea was able to ameliorate serum biochemical parameters in high sucrose stressed rabbits mediated by antioxidant and lipotropic properties. Keywords: Antioxidant, Anti-Atherosclerotic Effect, Hypoglycemic Effect, Rabbit 1. Introduction Kombucha tea is rich with nutritive properties, beneficial bacteria, multivitamins, enzymes and essential The history of Kombucha referred to a Korean physician organic acids such as acetic acid, lactic acid, folic acid, called Kombu, who was the first to introduce this gluconic acid, glucuronic acid, usnic acid, ascorbic acid beverage to Japanese importer (as a drink with healing and oxalic acid which helps liver in removing toxic properties). It is prepared by fermenting black tea with a substances6. special culture of yeasts and bacteria known as Kombucha Sucrose is a non-reducing disaccharide made up of mushroom. -

SUMMER SKIN TOOLKIT July9th 2020
SUMMER SKIN CAMPAIGN 2020 Live Date: JUNE 22, 2020 Updated: JULY 9, 2020 Product Overview Digital Display Banners E-Tailers · Product overview section · 300 x 600 px · QVC TABLE of · NEW Product Descriptions and PDP · 160 x 600 px · TSC · 728 x 90 px · Look Fantastic Main Creative · 468 x 60 px · NEXT CONTENTS · Primary Visuals · 320 x 50 px · Feelunique · Secondary Visuals · 320 x 100 px · Harvey Nichols · Supporting Visuals · 336 x 280 px · Debenhams · Digital Visuals · 300 x 250 px · Neiman Marcus · Video Storyboards · 1200 x 1200 px · Ulta · Nordstrom DIGITAL PRODUCT PAGE ASSETS Paid Social · Macy’s · Packshots, Alternative Images & Video · Instagram (Posts / Stories) · Articles / Infographic · Facebook · YouTube DIGITAL SITE ASSETS · Billboard (Desktop + Mobile) Owned Social · Secondary Billboard · Instagram (Posts / Stories) · Nav Promo/PLP Promo · Facebook · Blog Posts · Twitter · Landing Page · Youtube Emails · Email Guidelines · Email Templates · Email Examples Misc · Employee Email Signature PRODUCT OVERVIEW NEW PRODUCT DETAIL PAGE IMAGES Packshot Texture Image Holding Product Alt Hand Hero/Story Image VISION LA Agency: Still & Video Mandy Madden Kelley: Still & Video LUX STUDIO LONDON: Still & Video — — — Digital only rights 2 years Full print and digital rights in perpetuity Full print and digital rights in perpetuity SUPERFOOD AHA GLOW CLEANSING BUTTER — 840 x 840px Resolution 72dpi PRODUCT MERCHANDISING Primary Category — Meta Title Superfood AHA Glow Cleansing Butter Cleansers — Product Size 90ml, Travel 20ml — Recommended