Diet and Trophic Niche of Antarctic Silverfish Pleuragramma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species Status Assessment Emperor Penguin (Aptenodytes Fosteri)
SPECIES STATUS ASSESSMENT EMPEROR PENGUIN (APTENODYTES FOSTERI) Emperor penguin chicks being socialized by male parents at Auster Rookery, 2008. Photo Credit: Gary Miller, Australian Antarctic Program. Version 1.0 December 2020 U.S. Fish and Wildlife Service, Ecological Services Program Branch of Delisting and Foreign Species Falls Church, Virginia Acknowledgements: EXECUTIVE SUMMARY Penguins are flightless birds that are highly adapted for the marine environment. The emperor penguin (Aptenodytes forsteri) is the tallest and heaviest of all living penguin species. Emperors are near the top of the Southern Ocean’s food chain and primarily consume Antarctic silverfish, Antarctic krill, and squid. They are excellent swimmers and can dive to great depths. The average life span of emperor penguin in the wild is 15 to 20 years. Emperor penguins currently breed at 61 colonies located around Antarctica, with the largest colonies in the Ross Sea and Weddell Sea. The total population size is estimated at approximately 270,000–280,000 breeding pairs or 625,000–650,000 total birds. Emperor penguin depends upon stable fast ice throughout their 8–9 month breeding season to complete the rearing of its single chick. They are the only warm-blooded Antarctic species that breeds during the austral winter and therefore uniquely adapted to its environment. Breeding colonies mainly occur on fast ice, close to the coast or closely offshore, and amongst closely packed grounded icebergs that prevent ice breaking out during the breeding season and provide shelter from the wind. Sea ice extent in the Southern Ocean has undergone considerable inter-annual variability over the last 40 years, although with much greater inter-annual variability in the five sectors than for the Southern Ocean as a whole. -

Fishes of the Eastern Ross Sea, Antarctica
Polar Biol (2004) 27: 637–650 DOI 10.1007/s00300-004-0632-2 REVIEW Joseph Donnelly Æ Joseph J. Torres Tracey T. Sutton Æ Christina Simoniello Fishes of the eastern Ross Sea, Antarctica Received: 26 November 2003 / Revised: 16 April 2004 / Accepted: 20 April 2004 / Published online: 16 June 2004 Ó Springer-Verlag 2004 Abstract Antarctic fishes were sampled with 41 midwater in Antarctica is dominated by a few fish families and 6 benthic trawls during the 1999–2000 austral (Bathylagidae, Gonostomatidae, Myctophidae and summer in the eastern Ross Sea. The oceanic pelagic Paralepididae) with faunal diversity decreasing south assemblage (0–1,000 m) contained Electrona antarctica, from the Antarctic Polar Front to the continent (Ever- Gymnoscopelus opisthopterus, Bathylagus antarcticus, son 1984; Kock 1992; Kellermann 1996). South of the Cyclothone kobayashii and Notolepis coatsi. These were Polar Front, the majority of meso- and bathypelagic replaced over the shelf by notothenioids, primarily Ple- fishes have circum-Antarctic distributions (McGinnis uragramma antarcticum. Pelagic biomass was low and 1982; Gon and Heemstra 1990). Taken collectively, the concentrated below 500 m. The demersal assemblage fishes are significant contributors to the pelagic biomass was characteristic of East Antarctica and included seven and are important trophic elements, both as predators species each of Artedidraconidae, Bathydraconidae and and prey (Rowedder 1979; Hopkins and Torres 1989; Channichthyidae, ten species of Nototheniidae, and Lancraft et al. 1989, 1991; Duhamel 1998). Over the three species each of Rajidae and Zoarcidae. Common continental slope and shelf, notothenioids dominate the species were Trematomus eulepidotus (36.5%), T. scotti ichthyofauna (DeWitt 1970). Most members of this (32.0%), Prionodraco evansii (4.9%), T. -

Haswell Island (Haswell Island and Adjacent Emperor Penguin Rookery on Fast Ice)
Measure 5 (2016) Management Plan for Antarctic Specially Protected Area No. 127 Haswell Island (Haswell Island and Adjacent Emperor Penguin Rookery on Fast Ice) 1. Description of values to be protected The area includes Haswell Island with its littoral zone and adjacent fast ice when present. Haswell Island was discovered in 1912 by the Australian Antarctic Expedition led by D. Mawson. It was named after William Haswell, professor of biology who rendered assistance to the expedition. Haswell is the biggest island of the same-name archipelago, with a height of 93 meters and 0,82 sq.meters in area. The island is at 2,5 km distance from the Russian Mirny Station operational from 1956. At East and South-East of the island, there is a large colony of Emperor penguins (Aptenodytes forsteri) on fast ice. The Haswell Island is a unique breeding site for almost all breeding bird species in East Antarctica including the: Antarctic petrel (Talassoica antarctica), Antarctic fulmar (Fulmarus glacioloides), Cape petrel (Daption capense), Snow petrel (Pagodroma nivea), Wilson’s storm petrel (Oceanites oceanicus), South polar skua (Catharacta maccormicki), Lonnberg skua Catharacta antarctica lonnbergi and Adelie penguin (Pygoscelis adeliae). The Area supports five species of pinnipeds, including the Ross seal (Ommatophoca rossii) which falls in the protected species category. ATCM VIII (Oslo, 1975) approved its designation as SSSI 7 on the aforementioned grounds after a proposal by the USSR. Map 1 shows the location of the Haswell Islands (except Vkhodnoy Island), Mirny Station, and logistic activity sites. It was renamed and renumbered as ASPA No. 127 by Decision 1 (2002). -

Energetics of the Antarctic Silverfish, Pleuragramma Antarctica, from the Western Antarctic Peninsula
Chapter 8 Energetics of the Antarctic Silverfish, Pleuragramma antarctica, from the Western Antarctic Peninsula Eloy Martinez and Joseph J. Torres Abstract The nototheniid Pleuragramma antarctica, commonly known as the Antarctic silverfish, dominates the pelagic fish biomass in most regions of coastal Antarctica. In this chapter, we provide shipboard oxygen consumption and nitrogen excretion rates obtained from P. antarctica collected along the Western Antarctic Peninsula and, combining those data with results from previous studies, develop an age-dependent energy budget for the species. Routine oxygen consumption of P. antarctica fell in the midrange of values for notothenioids, with a mean of 0.057 ± −1 −1 0.012 ml O2 g h (χ ± 95% CI). P. antarctica showed a mean ammonia-nitrogen excretion rate of 0.194 ± 0.042 μmol NH4-N g−1 h−1 (χ ± 95% CI). Based on current data, ingestion rates estimated in previous studies were sufficient to cover the meta- bolic requirements over the year classes 0–10. Metabolism stood out as the highest energy cost to the fish over the age intervals considered, initially commanding 89%, gradually declining to 67% of the annual energy costs as the fish aged from 0 to 10 years. Overall, the budget presented in the chapter shows good agreement between ingested and combusted energy, and supports the contention of a low-energy life- style for P. antarctica, but it also resembles that of other pelagic species in the high percentage of assimilated energy devoted to metabolism. It differs from more tem- perate coastal pelagic fishes in its large investment in reproduction and its pattern of slow steady growth throughout a relatively long lifespan. -

Age and Growth of Brauer's Lanternfish Gymnoscopelus Braueri and Rhombic Lanternfish Krefftichthys Anderssoni (Family Myctophidae) in the Scotia Sea, Southern Ocean
Received: 22 July 2019 Accepted: 14 November 2019 DOI: 10.1111/jfb.14206 REGULAR PAPER FISH Age and growth of Brauer's lanternfish Gymnoscopelus braueri and rhombic lanternfish Krefftichthys anderssoni (Family Myctophidae) in the Scotia Sea, Southern Ocean Ryan A. Saunders1 | Silvia Lourenço2,3 | Rui P. Vieira4 | Martin A. Collins1 | Carlos A. Assis5,6 | Jose C. Xavier7,1 1British Antarctic Survey, Natural Environment Research Council, Cambridge, UK Abstract 2MARE - Marine and Environmental Sciences This study examines age and growth of Brauer's lanternfish Gymnoscopelus braueri and Centre, Polytechnic of Leiria, Peniche, Portugal rhombic lanternfish Krefftichthys anderssoni from the Scotia Sea in the Southern Ocean, 3CIIMAR/CIMAR, Interdisciplinary Centre of Marine and Environmental Research, through the analysis of annual growth increments deposited on sagittal otoliths. Oto- University of Porto, Matosinhos, Portugal lith pairs from 177 G. braueri and 118 K. anderssoni were collected in different seasons 4 Centre for Environment Fisheries and from the region between 2004 and 2009. Otolith-edge analysis suggested a seasonal Aquaculture Sciences, Lowestoft, UK change in opaque and hyaline depositions, indicative of an annual growth pattern, 5MARE-ULisboa – Marine and Environmental Sciences Centre, Faculty of Sciences, although variation within the populations of both species was apparent. Age estimates University of Lisbon, Lisbon, Portugal varied from 1 to 6 years for G. braueri (40 to 139 mm standard length; LS)andfrom 6Department of Animal Biology, Faculty of Sciences, University of Lisbon, Lisbon, Portugal 0 to 2 years for K. anderssoni (26 to 70 mm LS). Length-at-age data were broadly con- 7Department of Life Sciences, MARE-UC, sistent with population cohort parameters identified in concurrent length-frequency University of Coimbra, Coimbra, Portugal data from the region for both species. -

Along-Shelf Connectivity and Circumpolar Gene Flow in Antarctic
www.nature.com/scientificreports OPEN Along-shelf connectivity and circumpolar gene fow in Antarctic silverfsh (Pleuragramma Received: 16 March 2018 Accepted: 12 November 2018 antarctica) Published: xx xx xxxx Jilda Alicia Caccavo 1, Chiara Papetti1,2, Maj Wetjen3, Rainer Knust4, Julian R. Ashford5 & Lorenzo Zane1,2 The Antarctic silverfsh (Pleuragramma antarctica) is a critically important forage species with a circumpolar distribution and is unique among other notothenioid species for its wholly pelagic life cycle. Previous studies have provided mixed evidence of population structure over regional and circumpolar scales. The aim of the present study was to test the recent population hypothesis for Antarctic silverfsh, which emphasizes the interplay between life history and hydrography in shaping connectivity. A total of 1067 individuals were collected over 25 years from diferent locations on a circumpolar scale. Samples were genotyped at ffteen microsatellites to assess population diferentiation and genetic structuring using clustering methods, F-statistics, and hierarchical analysis of variance. A lack of diferentiation was found between locations connected by the Antarctic Slope Front Current (ASF), indicative of high levels of gene fow. However, gene fow was signifcantly reduced at the South Orkney Islands and the western Antarctic Peninsula where the ASF is absent. This pattern of gene fow emphasized the relevance of large-scale circulation as a mechanism for circumpolar connectivity. Chaotic genetic patchiness characterized population structure over time, with varying patterns of diferentiation observed between years, accompanied by heterogeneous standard length distributions. The present study supports a more nuanced version of the genetic panmixia hypothesis that refects physical-biological interactions over the life history. -

Antartic Peninsula and Tierra Del Fuego: 100
ANTARCTIC PENINSULA & TIERRA DEL FUEGO BALKEMA – Proceedings and Monographs in Engineering, Water and Earth Sciences Antarctic Peninsula & Tierra del Fuego: 100 years of Swedish-Argentine scientific cooperation at the end of the world Edited by Jorge Rabassa & María Laura Borla Proceedings of “Otto Nordenskjöld’s Antarctic Expedition of 1901–1903 and Swedish Scientists in Patagonia: A Symposium”, held in Buenos Aires, La Plata and Ushuaia, Argentina, March 2–7, 2003. LONDON / LEIDEN / NEW YORK / PHILADELPHIA / SINGAPORE Cover photo information: “The Otto Nordenskjöld’s Expedition to Antarctic Peninsula, 1901–1903. The wintering party in front of the hut on Snow Hill, Antarctica, 30th September 1902. From left to right: Bodman, Jonassen, Nordenskjöld, Ekelöf, Åkerlund and Sobral. Photo: G. Bodman. From the book: Otto Nordenskjöld & John Gunnar Andersson, et al., “Antarctica: or, Two Years amongst the Ice of the South Pole” (London: Hurst & Blackett., 1905)”. Taylor & Francis is an imprint of the Taylor & Francis Group, an informa business This edition published in the Taylor & Francis e-Library, 2007. “To purchase your own copy of this or any of Taylor & Francis or Routledge’s collection of thousands of eBooks please go to www.eBookstore.tandf.co.uk.” © 2007 Taylor & Francis Group plc, London, UK All rights reserved. No part of this publication or the information contained herein may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, by photocopying, recording or otherwise, without written prior permission from the publishers. Although all care is taken to ensure the integrity and quality of this publication and the information herein, no responsibility is assumed by the publishers nor the author for any damage to property or persons as a result of operation or use of this publication and/or the information contained herein. -

Main Drivers of Mercury Levels in Southern Ocean Lantern Fish Myctophidae
Main drivers of mercury levels in Southern Ocean Lantern fish Myctophidae José Seco, José Xavier, Paco Bustamante, Joao Coelho, Ryan Saunders, Nicole Ferreira, Sophie Fielding, Miguel Pardal, Gabriele Stowasser, Thainara Viana, et al. To cite this version: José Seco, José Xavier, Paco Bustamante, Joao Coelho, Ryan Saunders, et al.. Main drivers of mercury levels in Southern Ocean Lantern fish Myctophidae. Environmental Pollution, Elsevier, 2020, 264C (114711), 10.1016/j.envpol.2020.114711. hal-02570100 HAL Id: hal-02570100 https://hal.archives-ouvertes.fr/hal-02570100 Submitted on 11 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Main drivers of mercury levels in Southern Ocean Lantern fish Myctophidae José Seco*1,2, José C. Xavier3,4, Paco Bustamante5, João P. Coelho6, Ryan A. Saunders3, Nicole Ferreira1, Sophie Fielding3, Miguel A. Pardal8, Gabriele Stowasser3, Thainara Viana1, Geraint A. Tarling3, Eduarda Pereira1, Andrew S. Brierley2 1 Department of Chemistry and CESAM/REQUIMTE, University of Aveiro, 3810-193 Aveiro, Portugal 2 Pelagic -

Interannual Changes in Body Fat Condition Index of Minke Whales in the Antarctic
MARINE ECOLOGY PROGRESS SERIES Published December 17 Mar Ecol Prog Ser Interannual changes in body fat condition index of minke whales in the Antarctic Taro ~chii'~*,Narimasa Shinohara2, Yoshihiro ~ujise~,Shigetoshi Nishiwaki3, Koji ~atsuoka~ 'National Research Institute of Far Seas Fisheries, 5-7-1 Orido, Shimizu, 424-8633 Japan 2Faculty of Marine Science and Technology, Tokai University, 20-1, Orido, Shimizu, 424-8610 Japan 3The Institute of Cetacean Research, 4-18, Toyorni-cho, Chuo-ku, Tokyo 104-0055, Japan ABSTRACT To study whether or not wide-rang~ngpelagic predators should be affected by localized changes in prey availability, interannual variab~lityin body fat condition index (assessed from girth measurements) of minke whales Balaenoptera acutorostrata was analyzed in relation to their distribu- tion, stomach-content mass and sea-ice extent during the austral summer in the Antarctic Ocean between 130°E and 170°W. The research area comprised offshore, ice-edge and Ross Sea areas. Of the 3 years (1990/91. 1992/93 and 1994/95) included in the study, 1994/95 was a year of significantly poor body fat condition compared with the other 2 years. The 1994/95 year was characterized by extensive sea-ice conditions, covering the usually krill-rich slope region throughout the season. Since minke whales were scarce and their stomach-content mass small in the ice-edge area during 1994/95, food availability in the area during the season was considered to be poor as a result of the high sea-ice extent. Antarctic krill Euphausja superba was regularly the dominant prey species throughout the sur- vey area, although on the Ross Sea shelf E. -

Marine Ecology Progress Series 584:45
Vol. 584: 45–65, 2017 MARINE ECOLOGY PROGRESS SERIES Published December 7 https://doi.org/10.3354/meps12347 Mar Ecol Prog Ser OPENPEN ACCESSCCESS Distributions of krill and Antarctic silverfish and correlations with environmental variables in the western Ross Sea, Antarctica L. Brynn Davis1,*, Eileen E. Hofmann1, John M. Klinck1, Andrea Piñones2,3,4, Michael S. Dinniman1 1Center for Coastal Physical Oceanography, Old Dominion University, Norfolk, VA 23508, USA 2Instituto de Ciencias Marinas y Limnológicas, Universidad Austral de Chile, Casilla 567, Valdivia 5090000, Chile 3Centro de Investigación Dinámica de Ecosistemas Marinos de Altas Latitudes, Universidad Austral de Chile, Valdivia 5090000, Chile 4COPAS Sur-Austral, Universidad de Concepción, Concepción 4030000, Chile ABSTRACT: Antarctic krill Euphausia superba, crystal krill E. crystallorophias, and Antarctic sil- verfish Pleuragramma antarctica are key mid-trophic level species in the Ross Sea, connecting pri- mary production to the upper trophic levels. Distributions of these species were constructed from observations made in the western Ross Sea from 1988 to 2004. Distributions of environmental con- ditions were obtained from a 5-km resolution circulation model (temperature, mixed layer depth, surface speed) and satellite-derived observations (chlorophyll, sea ice cover). A hierarchy of sta- tistical methods determined correlations and relationships between species and environmental conditions. Each species occupies a localized habitat defined by different environmental charac- teristics. Antarctic krill are concentrated along the northwestern shelf break in a habitat charac- terized by deep (>1000 m) bottom depth, warm temperature (1 to 1.25°C), decreased sea ice, and proximity to the shelf break. Crystal krill and Antarctic silverfish are concentrated in Terra Nova Bay. -

1 Ainley.Pdf
55 MARINE ORNITHOLOGY Vol. 30 No. 2 2002 FORUM THE ROSS SEA, ANTARCTICA, WHERE ALL ECOSYSTEM PROCESSES STILL REMAIN FOR STUDY, BUT MAYBE NOT FOR LONG D.G. AINLEY H.T. Harvey & Associates, 3150 Almaden Expressway, Suite 145, San Jose, California 95118, USA ([email protected]) Received 29 October 2002, accepted 31 December 2002 SUMMARY AINLEY, D.G. 2002. The Ross Sea: where all ecosystem processes still remain for study, but maybe not for long. Marine Ornithology 30: 55–62. The Ross Sea is a well-defined embayment of Antarctica about the size of southern Europe, bounded by Victoria Land to the west, King Edward VII Peninsula, Marie Byrd Land to the east, the Ross Ice Shelf to the south, and the Pacific Sector of the Southern Ocean to the north. Its waters are composed of two related biotic systems: the Ross Sea Shelf Ecosystem (RSShelfE) and the Ross Sea Slope Ecosystem (RSSlopeE). The Ross Sea is off limits to mineral extraction, but pressures on its biological resources are growing. The economic value of the resources should be weighed against the value of the system as a unique scientific resource. The Ross Sea represents an unparal- leled natural laboratory in which the results of different fishery management strategies could be modeled in the context of short-term and decadal variation in biological populations, with these models applied throughout the Southern Ocean and elsewhere. The RSShelfE is the last Large Marine Ecosystem on Earth (except the Weddell Sea and, perhaps, Hudson Bay in the north of Canada) that has escaped direct anthropogenic alteration; the RSSlopeE, similar to all of Earth’s other marine ecosystems, has lost its large baleen whales but otherwise is intact. -
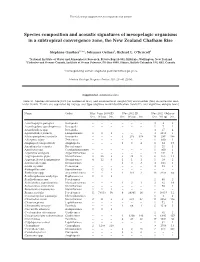
Marine Ecology Progress Series 503:23
The following supplement accompanies the article 1 Species composition and acoustic signatures of mesopelagic organisms in a subtropical convergence zone, the New Zealand Chatham Rise Stéphane Gauthier1, 2,*, Johannes Oeffner1, Richard L. O’Driscoll1 1National Institute of Water and Atmospheric Research, Private Bag 14-901, Kilbirnie, Wellington, New Zealand 2Fisheries and Oceans Canada, Institute of Ocean Sciences, PO Box 6000, Sidney, British Columbia V8L 4B2, Canada *Corresponding author: [email protected] Marine Ecology Progress Series: 503: 23–40 (2014) Supplement. Additional data Table S1. Species occurrence (Occ.) in number of tows, and abundance in weight (Wt) and number (No.) in successful mid - water trawls. Trawls are separated by voyage and type (daytime mark identification trawls ID, and nighttime oblique tows) Name Order May−June 2008 ID Nov 2011 ID Nov 2011 Oblique Occ. Wt (g) No. Occ. Wt (g) No. Occ. Wt (g) No. Acanthephyra pelagica Decapoda − − − − − − 3 9 3 Acanthephyra quadrispinosa Decapoda − − − − − − 1 7 1 Acanthephyra spp. Decapoda − − − − − − 3 17 4 Agrostichthys parkeri Lampriformes 1 8 1 − − − 3 1813 3 Alainopasiphaea australis Decapoda − − − 3 273 356 14 287 366 Allocyttus niger Zeiformes − − − − − − 1 600 1 Amphipod (unspecified) Amphipoda − − − 1 3 4 8 34 17 Anoplogaster cornuta Beryciformes − − − − − − 1 25 1 Apristurus spp. Carcharhiniformes − − − − − − 1 900 1 Argentina elongata Argentiniformes − − − − − − 4 541 6 Argyropelecus gigas Stomiiformes 2 20 2 2 38 5 8 165 12 Argyropelecus hemigymnus Stomiiformes 4 12 6 1 1 1 1 19 2 Astronesthes spp. Stomiiformes − − − 1 3 3 3 103 3 Atolla wyvillei Coronatae − − − − − − 4 83 7 Bathophilus ater Stomiiformes 1 12 1 − − − − − − Bathylagus spp.