Phylogenetic Position of the Enigmatic Termite Family Stylotermitidae (Insecta : Blattodea)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Ecology of Termite Nesting Behavior and Its Impact On
1 Evolution and Ecology of Termite Nesting Behavior and Its Impact on Disease Susceptibility A dissertation presented by Marielle Aimée Postava-Davignon to The Department of Biology In partial fulfillment of the requirements for the degree of Doctor of Philosophy in the field of Biology Northeastern University Boston, Massachusetts April, 2010 2 Evolution and Ecology of Termite Nesting Behavior and Its Impact on Disease Susceptibility by Marielle Aimée Postava-Davignon ABSTRACT OF DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology in the Graduate School of Arts and Sciences of Northeastern University, April, 2010 3 Abstract Termites construct nests that are often structurally species-specific. They exhibit a high diversity of nest structures, but their nest evolution is largely unknown. Current hypotheses for the factors that influenced nest evolution include adaptations that improved nest thermoregulation, defense against predators, and competition for limited nest sites. Studies have shown a lower prevalence of pathogens and parasites in arboreal nesting animal species compared to ground nesters. Nest building behavior is plastic and can adapt to changing environments. As termites can detect and avoid pathogens, I hypothesized that the evolution of arboreal termite nests was an adaptation to avoid infection. To test this, bacteria and fungi from nest cores, trails, and surrounding soils of the arboreal nesting Nasutitermes acajutlae were cultured. Abiotic factors such as temperature, relative humidity, and light were measured to elucidate how they influenced the interactions between termites and microbes. Fungi associated with N. acajutlae were identified to determine the potential pathogenic pressures these termites encounter in their nest as compared to the external environment. -

Symbiotic Cellulolytic Bacteria from the Gut of the Subterranean Termite Psammotermes Hypostoma Desneux and Their Role in Cellulose Digestion Huda R
Ali et al. AMB Expr (2019) 9:111 https://doi.org/10.1186/s13568-019-0830-5 ORIGINAL ARTICLE Open Access Symbiotic cellulolytic bacteria from the gut of the subterranean termite Psammotermes hypostoma Desneux and their role in cellulose digestion Huda R. K. Ali1*, Nada F. Hemeda2 and Yasser F. Abdelaliem3 Abstract The subterranean termite Psammotermes hypostoma Desneux is considered as an important pest that could cause severe damage to buildings, furniture, silos of grain and crops or any material containing cellulose. This species of termites is widespread in Egypt and Africa. The lower termite’s ability to digest cellulose depends on the association of symbiotic organisms gut that digest cellulose (fagellates and bacteria). In this study, 33 diferent bacterial isolates were obtained from the gut of the termite P. hypostoma which were collected using cellulose traps. Strains were grown on carboxymethylcellulose (CMC) as a sole source of carbon. Cellulolytic strains were isolated in two diferent cellulose medium (mineral salt medium containing carboxymethylcellulose as the sole carbon source and agar cel- lulose medium). Five isolates showed signifcant cellulolytic activity identifed by a Congo red assay which gives clear zone. Based on biochemical tests and sequencing of 16s rRNA genes these isolates were identifed as Paenibacillus lactis, Lysinibacillus macrolides, Stenotrophomonas maltophilia, Lysinibacillus fusiformis and Bacillus cereus, that depos- ited in GenBank with accession numbers MG991563, MG991564, MG991565, MG991566 and MG991567, respectively. Keywords: Termite, Psammotermes hypostoma, Symbiosis, Cellulose degrading bacteria, 16S rRNA gene, Phylogenetic analysis Introduction economic damage than food combined with fre (Eggle- Termites are social insects occurring in tropical, subtropical, ton 2011). -

Isoptera) in New Guinea 55 Doi: 10.3897/Zookeys.148.1826 Research Article Launched to Accelerate Biodiversity Research
A peer-reviewed open-access journal ZooKeys 148: 55–103Revision (2011) of the termite family Rhinotermitidae (Isoptera) in New Guinea 55 doi: 10.3897/zookeys.148.1826 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research Revision of the termite family Rhinotermitidae (Isoptera) in New Guinea Thomas Bourguignon1,2,†, Yves Roisin1,‡ 1 Evolutionary Biology and Ecology, CP 160/12, Université Libre de Bruxelles (ULB), Avenue F.D. Roosevelt 50, B-1050 Brussels, Belgium 2 Present address: Graduate School of Environmental Science, Hokkaido Uni- versity, Sapporo 060–0810, Japan † urn:lsid:zoobank.org:author:E269AB62-AC42-4CE9-8E8B-198459078781 ‡ urn:lsid:zoobank.org:author:73DD15F4-6D52-43CD-8E1A-08AB8DDB15FC Corresponding author: Yves Roisin ([email protected]) Academic editor: M. Engel | Received 19 July 2011 | Accepted 28 September 2011 | Published 21 November 2011 urn:lsid:zoobank.org:pub:27B381D6-96F5-482D-B82C-2DFA98DA6814 Citation: Bourguignon T, Roisin Y (2011) Revision of the termite family Rhinotermitidae (Isoptera) in New Guinea. In: Engel MS (Ed) Contributions Celebrating Kumar Krishna. ZooKeys 148: 55–103. doi: 10.3897/zookeys.148.1826 Abstract Recently, we completed a revision of the Termitidae from New Guinea and neighboring islands, record- ing a total of 45 species. Here, we revise a second family, the Rhinotermitidae, to progress towards a full picture of the termite diversity in New Guinea. Altogether, 6 genera and 15 species are recorded, among which two species, Coptotermes gambrinus and Parrhinotermes barbatus, are new to science. The genus Heterotermes is reported from New Guinea for the first time, with two species restricted to the southern part of the island. -
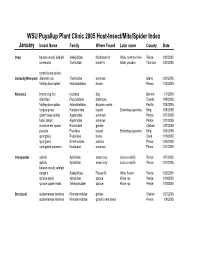
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

André Nel Sixtieth Anniversary Festschrift
Palaeoentomology 002 (6): 534–555 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ PALAEOENTOMOLOGY PE Copyright © 2019 Magnolia Press Editorial ISSN 2624-2834 (online edition) https://doi.org/10.11646/palaeoentomology.2.6.1 http://zoobank.org/urn:lsid:zoobank.org:pub:25D35BD3-0C86-4BD6-B350-C98CA499A9B4 André Nel sixtieth anniversary Festschrift DANY AZAR1, 2, ROMAIN GARROUSTE3 & ANTONIO ARILLO4 1Lebanese University, Faculty of Sciences II, Department of Natural Sciences, P.O. Box: 26110217, Fanar, Matn, Lebanon. Email: [email protected] 2State Key Laboratory of Palaeobiology and Stratigraphy, Center for Excellence in Life and Paleoenvironment, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China. 3Institut de Systématique, Évolution, Biodiversité, ISYEB-UMR 7205-CNRS, MNHN, UPMC, EPHE, Muséum national d’Histoire naturelle, Sorbonne Universités, 57 rue Cuvier, CP 50, Entomologie, F-75005, Paris, France. 4Departamento de Biodiversidad, Ecología y Evolución, Facultad de Biología, Universidad Complutense, Madrid, Spain. FIGURE 1. Portrait of André Nel. During the last “International Congress on Fossil Insects, mainly by our esteemed Russian colleagues, and where Arthropods and Amber” held this year in the Dominican several of our members in the IPS contributed in edited volumes honoring some of our great scientists. Republic, we unanimously agreed—in the International This issue is a Festschrift to celebrate the 60th Palaeoentomological Society (IPS)—to honor our great birthday of Professor André Nel (from the ‘Muséum colleagues who have given us and the science (and still) national d’Histoire naturelle’, Paris) and constitutes significant knowledge on the evolution of fossil insects a tribute to him for his great ongoing, prolific and his and terrestrial arthropods over the years. -

Evaluation of the Chemical Defense Fluids of Macrotermes Carbonarius
www.nature.com/scientificreports OPEN Evaluation of the chemical defense fuids of Macrotermes carbonarius and Globitermes sulphureus as possible household repellents and insecticides S. Appalasamy1,2*, M. H. Alia Diyana2, N. Arumugam2 & J. G. Boon3 The use of chemical insecticides has had many adverse efects. This study reports a novel perspective on the application of insect-based compounds to repel and eradicate other insects in a controlled environment. In this work, defense fuid was shown to be a repellent and insecticide against termites and cockroaches and was analyzed using gas chromatography-mass spectrometry (GC– MS). Globitermes sulphureus extract at 20 mg/ml showed the highest repellency for seven days against Macrotermes gilvus and for thirty days against Periplaneta americana. In terms of toxicity, G. sulphureus extract had a low LC50 compared to M. carbonarius extract against M. gilvus. Gas chromatography–mass spectrometry analysis of the M. carbonarius extract indicated the presence of six insecticidal and two repellent compounds in the extract, whereas the G. sulphureus extract contained fve insecticidal and three repellent compounds. The most obvious fnding was that G. sulphureus defense fuid had higher potential as a natural repellent and termiticide than the M. carbonarius extract. Both defense fuids can play a role as alternatives in the search for new, sustainable, natural repellents and termiticides. Our results demonstrate the potential use of termite defense fuid for pest management, providing repellent and insecticidal activities comparable to those of other green repellent and termiticidal commercial products. A termite infestation could be silent, but termites are known as destructive urban pests that cause structural damage by infesting wooden and timber structures, leading to economic loss. -

Treatise on the Isoptera of the World Kumar
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by American Museum of Natural History Scientific Publications KRISHNA ET AL.: ISOPTERA OF THE WORLD: 7. REFERENCES AND INDEX7. TREATISE ON THE ISOPTERA OF THE WORLD 7. REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA, AND MICHAEL S. ENGEL A MNH BULLETIN (7) 377 2 013 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY TREATISE ON THE ISOPTERA OF THE WORLD VolUME 7 REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192 AND MICHAEL S. ENGEL Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192; Division of Entomology (Paleoentomology), Natural History Museum and Department of Ecology and Evolutionary Biology 1501 Crestline Drive, Suite 140 University of Kansas, Lawrence, Kansas 66045 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 377, 2704 pp., 70 figures, 14 tables Issued April 25, 2013 Copyright © American Museum of Natural History 2013 ISSN 0003-0090 2013 Krishna ET AL.: ISOPtera 2435 CS ONTENT VOLUME 1 Abstract...................................................................... 5 Introduction.................................................................. 7 Acknowledgments . 9 A Brief History of Termite Systematics ........................................... 11 Morphology . 44 Key to the -

Taxonomy, Biogeography, and Notes on Termites (Isoptera: Kalotermitidae, Rhinotermitidae, Termitidae) of the Bahamas and Turks and Caicos Islands
SYSTEMATICS Taxonomy, Biogeography, and Notes on Termites (Isoptera: Kalotermitidae, Rhinotermitidae, Termitidae) of the Bahamas and Turks and Caicos Islands RUDOLF H. SCHEFFRAHN,1 JAN KRˇ ECˇ EK,1 JAMES A. CHASE,2 BOUDANATH MAHARAJH,1 3 AND JOHN R. MANGOLD Ann. Entomol. Soc. Am. 99(3): 463Ð486 (2006) ABSTRACT Termite surveys of 33 islands of the Bahamas and Turks and Caicos (BATC) archipelago yielded 3,533 colony samples from 593 sites. Twenty-seven species from three families and 12 genera were recorded as follows: Cryptotermes brevis (Walker), Cr. cavifrons Banks, Cr. cymatofrons Schef- Downloaded from frahn and Krˇecˇek, Cr. bracketti n. sp., Incisitermes bequaerti (Snyder), I. incisus (Silvestri), I. milleri (Emerson), I. rhyzophorae Herna´ndez, I. schwarzi (Banks), I. snyderi (Light), Neotermes castaneus (Burmeister), Ne. jouteli (Banks), Ne. luykxi Nickle and Collins, Ne. mona Banks, Procryptotermes corniceps (Snyder), and Pr. hesperus Scheffrahn and Krˇecˇek (Kalotermitidae); Coptotermes gestroi Wasmann, Heterotermes cardini (Snyder), H. sp., Prorhinotermes simplex Hagen, and Reticulitermes flavipes Koller (Rhinotermitidae); and Anoplotermes bahamensis n. sp., A. inopinatus n. sp., Nasuti- termes corniger (Motschulsky), Na. rippertii Rambur, Parvitermes brooksi (Snyder), and Termes http://aesa.oxfordjournals.org/ hispaniolae Banks (Termitidae). Of these species, three species are known only from the Bahamas, whereas 22 have larger regional indigenous ranges that include Cuba, Florida, or Hispaniola and beyond. Recent exotic immigrations for two of the regional indigenous species cannot be excluded. Three species are nonindigenous pests of known recent immigration. IdentiÞcation keys based on the soldier (or soldierless worker) and the winged imago are provided along with species distributions by island. Cr. bracketti, known only from San Salvador Island, Bahamas, is described from the soldier and imago. -

Discovery of a Cryptic Termite Genus, Stylotermes (Isoptera: Stylotermitidae), in Taiwan, with the Description of a New Species
Annals of the Entomological Society of America, 110(4), 2017, 360–373 doi: 10.1093/aesa/sax034 Advance Access Publication Date: 23 March 2017 Research Research article Discovery of a Cryptic Termite Genus, Stylotermes (Isoptera: Stylotermitidae), in Taiwan, With the Description of a New Species Wei-Ren Liang, Chia-Chien Wu, and Hou-Feng Li1 Department of Entomology, National Chung Hsing University, 145 Xingda Rd., Taichung 40227, Taiwan ([email protected]; [email protected]; [email protected]), and 1Corresponding author, e-mail: [email protected] Subject Editor: Allen Szalanski Received 18 October 2016; Editorial decision 11 January 2017 Abstract The termite family Stylotermitidae consists of a single extant genus, Stylotermes, present only in eastern Asia. Stylotermes has distinctively trimerous tarsi and is considered an intermediate between the Rhinotermitidae and Kalotermitidae families. The present study reports the first discovery of the Stylotermitidae family in Taiwan. On the basis of a comparison between the Taiwanese samples and the original descriptions of all the other 44 Stylotermes species, a new species collected from eastern Taiwan is described as Stylotermes halumi- cus sp. nov. This study is the first to report the gene sequence and detailed morphological descriptions of the winged imago, soldier, and worker of a single Stylotermes sp. Furthermore, a preliminary review of the Stylotermes taxonomy is provided. Key words: Stylotermitidae, wood-feeding termite, Taiwan, 16S, COII Holmgren and Holmgren (1917) established a new genus, Malaysia. In China, 35 Stylotermes spp. were described between Stylotermes, as the type genus of the new Stylotermitinae subfamily 1963 and 1993 from eight provinces: Fujian [no. -

Sultan Qaboos University Journal for Scientific Research
Agricultural and Marine Sciences, 10(1):33-40 (2005) ©2005 Sultan Qaboos University Identification, Geographical Distribution and Hosts of Subterranean Termites in the United Arab Emirates Arid Ecosystem W. Kaakeh Department of Arid Land Agriculture, College of Food Systems, P. O. Box 17555, United Arab Emirates University, Al-Ain, United Arab Emirates وﻟﯿﺪ ﻛﻌﻚ اﻟﺨﻼﺻﺔ: ﺗﻢ ﺗﻌﺮﻳﻒ ﺳﺘﺔ أﻧﻮاع ﻣﻦ اﻟﻨﻤﻞ اﻷﺑﯿﺾ (اﻷرﺿﺔ) ﺗﺎﺑﻌﺔ إﻟﻰ ﺧﻤﺴﺔ أﺟﻨﺎس وﺛﻼث ﻓﺼﺎﺋﻞ (ھﻮدوjرﻣﯿﺘﯿﺪي Hodotermitidae، راﻳﻨﻮﺗﺮﻣﯿﺘﯿﺪي Rhinotermi،Rhinotermitidaetidae، وﺗﺮﻣﯿﺘﯿﺪي T(Termitidaeermitidae) ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وأﻧﻮاع اﻷرﺿﺔ اﻟﺘﻲ ﺗﻢ ﺗﺴﺠﯿﻠﮭﺎ ھﻲ اﻷرﺿﺔ اﻟﺤﺎﺻﺪة أو اﻷرﺿﺔ اﻟﻼﺷﻮﻛﯿﺔ Anacanthotermes ochraceusochraceus (Burmeister(Burmeister) و Anacanthotermes ubachi (Navas(Navas)، وأرﺿﺔ اﻟﺮﻣﻞ اﻟﺜﻐﺮﻳﺔ Psammotermes hypostomahypostoma (Desneux)، واﻷرﺿﺔ اﻟﺸﻤﻌﯿﺔ اﻟﺼﻐﯿﺮة MicrocerotermesMicrocerotermes diversusdiversus Silvestri))، واﻷرﺿﺔ اﻟﻨﺠﺪﻳﺔ اﻟﺪﻗﯿﻘﺔ Microtermes najdensis (Harris) ، واﻷرﺿﺔ Heterotermes aethiopicus (Sjostedt)، وﺑﺎﺳﺘﺜﻨﺎء اﻟﻨﻮع H. aethiopicus، ﻓﺈﻧﻪ ﺗﻢ ﺗﺴﺠﯿﻞ اﻷﻧﻮاع اﻟﺨﻤﺴﺔ اﻷﺧﺮى ﻟﻠﻤﺮة اﻷوﻟﻰ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وﺗﻌﯿﺶ ﻛﻞ اﻷﻧﻮاع ﺗﺤﺖ اﻷرض وﺗﺼﻞ إﻟﻰ ﻣﺼﺎدر اﻟﻐﺬاء اﻟﺨﺸﺒﯿﺔ ﻣﻦ ﺧﻼل أﻧﻈﻤﺔ اﻷﻧﻔﺎق اﻟﻄﯿﻨﯿﺔ. وﻗﺪ وﺟﺪت اﻷرﺿﺔ ﻓﻲ ﻣﻨﺎﻃﻖ ﻣﺨﺘﻠﻔﺔ ﻣﻦ اﻟﺪوﻟﺔ واﻟﺘﻲ ﺗﺘﻤﯿﺰ ﺑﺎﺧﺘﻼف ﻇﺮوﻓﮭﺎ اﻟﻤﻨﺎﺧﯿﺔ وﻏﻄﺎءھﺎ اﻟﻨﺒﺎﺗﻲ وﻧﻮع ﺗﺮﺑﺘﮭﺎ. وﺗﻔﻀﻞ اﻷرﺿﺔ اﻟﺘﻐﺬﻳﺔ ﻋﻠﻰ اﻟﻌﻮاﺋﻞ اﻟﺤﯿﺔ أو اﻟﻤﯿﺘﺔ أو اﻟﻤﺘﻌﻔﻨﺔ، ﺑﺎﻹﺿﺎﻓﺔ إﻟﻰ اﻟﻤﻮاد ﻏﯿﺮ اﻟﺴﯿﻠﯿﻠﻮزﻳﺔ. وﻣﻦ أﻛﺜﺮ أﻧﻮاع اﻷرﺿﺔ ًﺗﻮزﻳﻌﺎ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة ھﻲ A. ochraceusochraceus وﺗﺘﺒﻌﮭﺎ ﻛﻞ ﻣﻦ P.P. hypostomahypostoma وdiversusوM. diversus. وﻗﺪ اﺧﺘﻠﻒ ﺗﻮزﻳﻊ اﻷﻧﻮاع اﻟﺴﺘﺔ ﺿﻤﻦ -

Termites (Isoptera) in the Azores: an Overview of the Four Invasive Species Currently Present in the Archipelago
Arquipelago - Life and Marine Sciences ISSN: 0873-4704 Termites (Isoptera) in the Azores: an overview of the four invasive species currently present in the archipelago MARIA TERESA FERREIRA ET AL. Ferreira, M.T., P.A.V. Borges, L. Nunes, T.G. Myles, O. Guerreiro & R.H. Schef- frahn 2013. Termites (Isoptera) in the Azores: an overview of the four invasive species currently present in the archipelago. Arquipelago. Life and Marine Sciences 30: 39-55. In this contribution we summarize the current status of the known termites of the Azores (North Atlantic; 37-40° N, 25-31° W). Since 2000, four species of termites have been iden- tified in the Azorean archipelago. These are spreading throughout the islands and becoming common structural and agricultural pests. Two termites of the Kalotermitidae family, Cryp- totermes brevis (Walker) and Kalotermes flavicollis (Fabricius) are found on six and three of the islands, respectively. The other two species, the subterranean termites Reticulitermes grassei Clemént and R. flavipes (Kollar) of the Rhinotermitidae family are found only in confined areas of the cities of Horta (Faial) and Praia da Vitória (Terceira) respectively. Due to its location and weather conditions the Azorean archipelago is vulnerable to coloni- zation by invasive species. The fact that there are four different species of termites in the Azores, all of them considered pests, is a matter of concern. Here we present a comparative description of these species, their known distribution in the archipelago, which control measures are being used against them, and what can be done in the future to eradicate and control these pests in the Azores.