Lactulose: an Effective Preventive and Therapeutic Option for Ischemic Stroke by Production of Hydrogen Xiao Chen1, Xiao Zhai2, Zhimin Kang3 and Xuejun Sun3*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Intake of Food Hydrocolloids of Bacterial Origin on the Glycemic Response in Humans: Systematic Review and Narrative Synthesis
nutrients Review Effect of Intake of Food Hydrocolloids of Bacterial Origin on the Glycemic Response in Humans: Systematic Review and Narrative Synthesis Norah A. Alshammari 1,2, Moira A. Taylor 3, Rebecca Stevenson 4 , Ourania Gouseti 5, Jaber Alyami 6 , Syahrizal Muttakin 7,8, Serafim Bakalis 5, Alison Lovegrove 9, Guruprasad P. Aithal 2 and Luca Marciani 2,* 1 Department of Clinical Nutrition, College of Applied Medical Sciences, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia; [email protected] 2 Translational Medical Sciences and National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre, Nottingham University Hospitals NHS Trust and University of Nottingham, Nottingham NG7 2UH, UK; [email protected] 3 Division of Physiology, Pharmacology and Neuroscience, School of Life Sciences, Queen’s Medical Centre, National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre, Nottingham NG7 2UH, UK; [email protected] 4 Precision Imaging Beacon, University of Nottingham, Nottingham NG7 2UH, UK; [email protected] 5 Department of Food Science, University of Copenhagen, DK-1958 Copenhagen, Denmark; [email protected] (O.G.); [email protected] (S.B.) 6 Department of Diagnostic Radiology, Faculty of Applied Medical Science, King Abdulaziz University (KAU), Jeddah 21589, Saudi Arabia; [email protected] 7 Indonesian Agency for Agricultural Research and Development, Jakarta 12540, Indonesia; Citation: Alshammari, N.A.; [email protected] Taylor, M.A.; Stevenson, R.; 8 School of Chemical Engineering, University of Birmingham, Birmingham B15 2TT, UK Gouseti, O.; Alyami, J.; Muttakin, S.; 9 Rothamsted Research, Harpenden, Hertfordshire AL5 2JQ, UK; [email protected] Bakalis, S.; Lovegrove, A.; Aithal, G.P.; * Correspondence: [email protected]; Tel.: +44-115-823-1248 Marciani, L. -

Differential Effects of the Poly (ADP-Ribose)Polymerase (PARP
British Journal of Cancer (2001) 84(1), 106–112 © 2001 Cancer Research Campaign doi: 10.1054/ bjoc.2000.1555, available online at http://www.idealibrary.com on http://www.bjcancer.com Differential effects of the poly (ADP-ribose) polymerase (PARP) inhibitor NU1025 on topoisomerase I and II inhibitor cytotoxicity in L1210 cells in vitro KJ Bowman*, DR Newell, AH Calvert and NJ Curtin Cancer Research Unit, University of Newcastle upon Tyne Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, UK Summary The potent novel poly(ADP-ribose) polymerase (PARP) inhibitor, NU1025, enhances the cytotoxicity of DNA-methylating agents and ionizing radiation by inhibiting DNA repair. We report here an investigation of the role of PARP in the cellular responses to inhibitors of topoisomerase I and II using NU1025. The cytotoxicity of the topoisomerase I inhibitor, camptothecin, was increased 2.6-fold in L1210 cells by co-incubation with NU1025. Camptothecin-induced DNA strand breaks were also increased 2.5-fold by NU1025 and exposure to camptothecin-activated PARP. In contrast, NU1025 did not increase the DNA strand breakage or cytotoxicity caused by the topoisomerase II inhibitor etoposide. Exposure to etoposide did not activate PARP even at concentrations that caused significant levels of apoptosis. Taken together, these data suggest that potentiation of camptothecin cytotoxicity by NU1025 is a direct result of increased DNA strand breakage, and that activation of PARP by camptothecin-induced DNA damage contributes to its repair and consequently cell survival. However, in L1210 cells at least, it would appear that PARP is not involved in the cellular response to etoposide-mediated DNA damage. -

(12) Patent Application Publication (10) Pub. No.: US 2012/0028333 A1 Piatesi Et Al
US 20120028333A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0028333 A1 Piatesi et al. (43) Pub. Date: Feb. 2, 2012 (54) USE OF ENZYMES TO REDUCE ALDEHYDES (30) Foreign Application Priority Data FROMALDEHYDE-CONTAINING PRODUCTS Apr. 7, 2009 (EP) .................................. O9157522.5 Publication Classification (76) Inventors: Andrea Piatesi, Mannheim (DE); (51) Int. Cl. Tilo Habicher, Speyer (DE); CI2N 9/02 (2006.01) Michael Bischel, Worms (DE); CI2N I/00 (2006.01) Li-Wen Wang, Mannheim (DE): CI2N 15/63 (2006.01) Jirgen Reichert, Limburgerhof A62D 3/02 (2007.01) (DE); Rainer Packe-Wirth, C7H 2L/04 (2006.01) Trostberg (DE); Kai-Uwe (52) U.S. Cl. ... 435/189: 435/262:536/23.2:435/320.1; Baldenius, Heidelberg (DE); Erich 435/243 Kromm, Weisenheim am Sand (57) ABSTRACT (DE); Stefan Häfner, Speyer (DE); Carsten Schwalb. Mannheim (DE); The invention relates to the use of an enzyme preparation Hans Wolfgang Höffken, which catalyzes the degradation of formaldehyde for reduc Ludwigshafen (DE) ing the formaldehyde content in a formaldehyde-containing formulation. In a preferred embodiment, the enzyme prepa ration contains a formaldehyde dismutase from a Pseudomo (21) Appl. No.: 13/262,662 nas putida Strain. Further, the invention refers to a process for reducing the formaldehyde content in cross-linking agents for textile finishing or in polymer dispersions used, e.g. in con (22) PCT Filed: Mar. 31, 2010 struction chemistry. Further the invention relates to the use of an enzyme preparation which catalyzes the degradation of (86). PCT No.: PCT/EP1OAS4284 aldehydes for reducing the formaldehyde content in an alde hyde-containing formulation. -

The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide As Batch Reaction
Pak. J. Chem. 5(3): 24-30, 2016 Full Paper ISSN (Print): 2220-2625 ISSN (Online): 2222-307X DOI: 10.15228/2016.v06.i01-2.p04 The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide as Batch Reaction *Nahla T. K. Department of Food Sciences, College of Agriculture, University of Baghdad, Iraq E-mail: *[email protected] ABSTRACT Lactulose is a synthetic and non-absorbable disaccharide sugar containing glucose and fructose units. It is used for the treatment of constipation and hepatic encephalopathy. The purpose of this experiment was to synthesized lactulose in the presence of sodium hydroxide as a catalyst. The evaluation of lactulose synthesis was carried out at different concentration of sodium hydroxide (1, 2 and 3M) and the study was also conducted at different temperatures (30, 50, 70, and 90 °C). In addition, the kinetics of lactose isomerization with respect to the time also studied. Finally, it was concluded that the lactulose formation was determined by spectrophotometric method. The lactulose isomerization increased about more than 30.9% at 90 °C in the presence of 1M sodium hydroxide and 20 % lactose concentration. Keywords: Lactose, isomerization, lactulose, sodium hydroxide. 1. INTRODUCTION Lactulose is a synthetic sugar, which is not adsorbed by humans or rodents due to the lack of enzymes which catalyzes the disaccharide sugars. It can be generated by either alkaline isomerization of lactose via the Lobry de Bruyn - Alberda van Ekenstein rearrangement or by enzyme-catalyzed synthesis. Based on the first reaction, different process schemes for the preparation of lactulose have been developed. The enzymatic synthesis of lactulose can be carried out using different pathways with the transgalactosylation reaction being the most promising1. -

Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles
pharmaceutics Article Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles Timothy M. Amis, Jwala Renukuntla, Pradeep Kumar Bolla and Bradley A. Clark * Department of Basic Pharmaceutical Sciences, Fred Wilson School of Pharmacy, High Point University, High Point, NC 27268, USA; [email protected] (T.M.A.); [email protected] (J.R.); [email protected] (P.K.B.) * Correspondence: [email protected]; Tel.: +1-336-841-9665 Received: 18 August 2020; Accepted: 16 September 2020; Published: 19 September 2020 Abstract: Cryoprotectants are often required in lyophilization to reduce or eliminate agglomeration of solute or suspended materials. The aim of this study was to select a cryoprotecting agent and optimize its concentration in a solid lipid nanoparticle formulation. Progesterone-loaded stearic acid solid lipid nanoparticles (SA-P SLNs) were prepared by hot homogenization with high speed mixing and sonication. The stearic acid content was 4.6% w/w and progesterone was 0.46% w/w of the initial formulation. Multiple surfactants were evaluated, and a lecithin and sodium taurocholate system was chosen. Three concentrations of surfactant were then evaluated, and a concentration of 2% w/w was chosen based on particle size, polydispersity, and zeta potential. Agglomeration of SA-P SLNs after lyophilization was observed as measured by increased particle size. Dextran, glycine, mannitol, polyvinylpyrrolidone (PVP), sorbitol, and trehalose were evaluated as cryoprotectants by both an initial freeze–thaw analysis and after lyophilization. Once selected as the cryoprotectant, trehalose was evaluated at 5%, 10%, 15%, and 20% for optimal concentration, with 20% trehalose being finally selected as the level of choice. -

Concentration and Distribution of Sialic Acid in Human Milk and Infant Formulas1–3
Concentration and distribution of sialic acid in human milk and infant formulas1–3 Bing Wang, Janette Brand-Miller, Patricia McVeagh, and Peter Petocz Downloaded from https://academic.oup.com/ajcn/article/74/4/510/4737459 by guest on 23 September 2021 ABSTRACT 2- to 4-fold higher than those of other mammals, including chim- Background: In animal studies, sialic acid supplementation is panzees (4). Sialic acid is thought to play a role in the structural associated with increases of gangliosides in the brain and and functional establishment of synaptic pathways: >40% of improved learning ability. Only limited data are available on the sialic acid in the brain is found in the synaptosomal fraction and sialic acid content of human milk and infant formulas. contributes to the negative charge of the membrane (3). Because Objective: We compared the concentrations of oligosaccharide- most neurotransmitters are positively charged, sialic acid may bound, protein-bound, and free sialic acid in milk from mothers of assist neurotransmission by facilitating the binding of transmit- full-term and preterm infants and in a range of infant formulas. ter molecules to the synaptic membrane. Design: The milk from 20 and 14 mothers of full-term and Morgan and Winick (2) showed that exogenous sialic acid preterm infants (mean gestational age: 31 ± 3 wk), respectively, administered by intraperitoneal injection increased the produc- was collected at 4 stages of lactation (colostrum, transition, 1 mo, tion of ganglioside sialic acid in the brain and improved learning and 3 mo) and compared with 21 different infant formulas. ability in well-nourished and malnourished rat pups. -

Xorox Univerelty Microfilms
INFORMATION TO USERS This material was producad from a microfilm copy of the original document. While the moit advanced technological meant to photograph and reproduce thii document have been used, the quality it heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help ou understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to ob tain the mining page(s) or section, they are spliced into the film along w ith: adjacent pages, This may have necessitated cutting thru an image and dupli eating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image, fou will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning” the material. It is customary to begin photoi ig at the upper left hand comer of a large dieet and to continue photoi ig from left to right in equal sections with a small overlap. If necessary, sectioning is continued agein — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Lactulose Solution, Usp
LACTULOSE- lactulose solution VistaPharm, Inc ---------- LACTULOSE SOLUTION, USP Rx Only VP2051 04/10 DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g of other sugars). Also contains FD&C Yellow No. 6, purified water, USP and wild cherry flavoring. A minimal quantity of sodium hydroxide, NF is used to adjust pH when necessary. The pH range is 2.5 to 6.5. Lactulose is a colonic acidifier which promotes laxation.The chemical name for lactulose is 4-O-β-D- galactopyranosyl-D-fructofuranose. It has the following structural formula: C12H22O11 The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. -

6) Dextran Antibody → Behavior of an Anti
Position Effects of Variable Region Carbohydrate on the Affinity and In Vivo Behavior of an Anti-(1→6) Dextran Antibody This information is current as M. Josefina Coloma, Ryan K. Trinh, Alexander R. Martinez of September 27, 2021. and Sherie L. Morrison J Immunol 1999; 162:2162-2170; ; http://www.jimmunol.org/content/162/4/2162 Downloaded from References This article cites 45 articles, 14 of which you can access for free at: http://www.jimmunol.org/content/162/4/2162.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 27, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 1999 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Position Effects of Variable Region Carbohydrate on the Affinity and In Vivo Behavior of an Anti-(136) Dextran Antibody1 M. Josefina Coloma, Ryan K. Trinh, Alexander R. Martinez, and Sherie L. Morrison2 IgG is a glycoprotein with an N-linked carbohydrate structure attached to the CH2 domain of each of its heavy chains. -
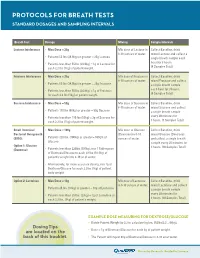
Protocols for Breath Tests Interpretation Help Standard Dosages and Sampling Intervals for Hydrogen/Methane Breath Tests
PROTOCOLS FOR BREATH TESTS INTERPRETATION HELP STANDARD DOSAGES AND SAMPLING INTERVALS FOR HYDROGEN/METHANE BREATH TESTS Breath Test Dosage Mixing Sample Intervals LACTOSE, FRUCTOSE OR SUCROSE INTOLERANCE: Lactose Intolerance • Max Dose = 25g Mix dose of Lactose in Collect Baseline, drink SUSPECTED POSITIVE: 8-10 ounces of water. mixed Lactose and collect a • Hydrogen (H ) Production Only: 20 ppm delta increase in Hydrogen from the lowest preceding value in the test. • Patients 55 lbs (24.9kg) or greater = 25g Lactose single breath sample each 2 hour for 3 hours. • Patients less than 55 lbs (24.9kg) = 1g of Lactose for • Methane (CH4) Production Only: 12 ppm delta increase in Methane from the lowest preceding value in the test. (4 Samples Total) each 2.2 lbs (1kg) of patient weight. • Hydrogen/Methane Both Produced: Add both H2 and CH4 values together in each sample. Review for a 15 ppm delta increase from the lowest preceding value. Fructose Intolerance • Max Dose = 25g Mix dose of Fructose in Collect Baseline, drink 8-10 ounces of water. mixed Fructose and collect • Patients 55 lbs (24.9kg) or greater = 25g Fructose a single breath sample each hour for 3 hours. SMALL INTESTINAL BACTERIAL OVERGROWTH (SIBO): • Patients less than 55 lbs (24.9kg) = 1g of Fructose (4 Samples Total) for each 2.2 lbs (1kg) of patient weight. SUSPECTED POSITIVE: Sucrose Intolerance • Max Dose = 50g Mix dose of Sucrose in Collect Baseline, drink OPTION 1: GLUCOSE (DEXTROSE) 8-10 ounces of water. mixed Sucrose and collect • Patients 110 lbs (50kg) or greater = 50g Sucrose • Hydrogen (H ) Production Only: 12 ppm delta increase in a single breath sample 2 every 30 minutes for Hydrogen from the lowest preceding value in the test. -

REVIEW the Role and Potential of Sialic Acid in Human Nutrition
European Journal of Clinical Nutrition (2003) 57, 1351–1369 & 2003 Nature Publishing Group All rights reserved 0954-3007/03 $25.00 www.nature.com/ejcn REVIEW The role and potential of sialic acid in human nutrition B Wang1* and J Brand-Miller1 1Human Nutrition Unit, School of Molecular and Microbial Biosciences, University of Sydney, NSW, Australia Sialic acids are a family of nine-carbon acidic monosaccharides that occur naturally at the end of sugar chains attached to the surfaces of cells and soluble proteins. In the human body, the highest concentration of sialic acid (as N-acetylneuraminic acid) occurs in the brain where it participates as an integral part of ganglioside structure in synaptogenesis and neural transmission. Human milk also contains a high concentration of sialic acid attached to the terminal end of free oligosaccharides, but its metabolic fate and biological role are currently unknown. An important question is whether the sialic acid in human milk is a conditional nutrient and confers developmental advantages on breast-fed infants compared to those fed infant formula. In this review, we critically discuss the current state of knowledge of the biology and role of sialic acid in human milk and nervous tissue, and the link between sialic acid, breastfeeding and learning behaviour. European Journal of Clinical Nutrition (2003) 57, 1351–1369. doi:10.1038/sj.ejcn.1601704 Keywords: sialic acid; ganglioside; sialyl-oligosaccharides; human milk; infant formula; breastfeeding Introduction promising new candidate is sialic acid (also known as The rapid growth and development of the newborn infant N-acetylneuraminic acid), a nine-carbon sugar that is a puts exceptional demands on the supply of nutrients. -

LACTULOSE SOLUTION USP - Lactulose Solution Hi-Tech Pharmacal Co., Inc
LACTULOSE SOLUTION USP - lactulose solution Hi-Tech Pharmacal Co., Inc. DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g or less of other sugars). Lactulose solution contains potassium sorbate as an inactive ingredient. Lactulose is a colonic acidifier which promotes laxation. The chemical name for lactulose is 4-O-β-D-galactopyranosyl-D-fructofuranose. It has the following structural formula: The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. Lactulose given orally to man and experimental animals resulted in only small amounts reaching the blood. Urinary excretion has been determined to be 3% or less and is essentially complete within 24 hours.