Polymorphism and Partial Characterization of Digestive Enzymes in the Spiny Lobster Panulirus Argus Comparative Biochemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

History of Alaska Red King Crab, Paralithodes Camtschaticus, Bottom Trawl Surveys, 1940–61
History of Alaska Red King Crab, Paralithodes camtschaticus, Bottom Trawl Surveys, 1940–61 MARK ZIMMERMANN, C. BRAXTON DEW, and BEVERLY A. MALLEY Introduction As early as the 1930’s, the Alaska ≥135 mm) was recorded in 1980. Then, red king crab resource was a heav- in 1981, the Bristol Bay red king crab The U.S. government was integrally ily exploited species, with significant population abruptly collapsed in one involved with the development of Alas- foreign commercial harvests occurring of the more precipitous declines in the ka’s red king crab, Paralithodes camts- well before the first U.S. (1940–41) ex- history of U.S. fisheries management. chaticus, fishing industry (Blackford, ploratory bottom trawl survey (Schmitt, In the opinion of Alaska crab manag- 1979). In 1940 Congress appropriated 1940; FWS, 1942). During the 1930’s, ers and modelers (Otto, 1986; Zheng funds for surveys of Alaska’s fishery Japan and Russia together took 9–14 and Kruse, 2002; NPFMC1), the crash resources (Schmitt, 1940; FWS, 1942). million kg of king crab per year from of the Bristol Bay red king crab stock Based on the U.S. Fish and Wildlife the southeast Bering Sea, within the vast was a natural phenomenon unrelated to Service’s (FWS) exploratory work for area referred to as Bristol Bay. commercial fishing. red king crab during the 1940’s, skepti- In 1946, U.S. fishermen began com- Considering the history of the Bristol cal commercial trawlers expected the mercial king crab fishing in these same Bay red king crab commercial fishery bulk of their profits to come from bot- waters, and by 1963 the United States and the unresolved issues surrounding tomfish. -

2005 Bottom Trawl Survey of the Eastern Bering Sea Continental Shelf
Alaska Fisheries Science Center National Marine Fisheries Service U.S DEPARTMENT OF COMMERCE AFSC PROCESSED REPORT 2007-01 2005 Bottom Trawl Survey of the Eastern Bering Sea Continental Shelf January 2007 This report does not constitute a publication and is for information only. All data herein are to be considered provisional. This document should be cited as follows: Lauth, R, and E. Acuna (compilers). 2007. 2005 bottom trawl survey of the eastern Bering Sea continental shelf. AFSC Processed Rep. 2007-1, 164 p. Alaska Fish. Sci. Cent., NOAA, Natl. Mar, Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. Notice to Users of this Document This document is being made available in .PDF format for the convenience of users; however, the accuracy and correctness of the document can only be certified as was presented in the original hard copy format. 2005 BOTTOM TRAWL SURVEY OF THE EASTERN BERING SEA CONTINENTAL SHELF Compilers Robert Lauth Erika Acuna Bering Sea Subtask Erika Acuna Lyle Britt Jason Conner Gerald R. Hoff Stan Kotwicki Robert Lauth Gary Mundell Daniel Nichol Duane Stevenson Ken Weinberg Resource Assessment and Conservation Engineering Division Alaska Fisheries Science Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 7600 Sand Point Way N.E. Seattle, WA 98115-6349 January 2007 ABSTRACT The Resource Assessment and Conservation Engineering Division of the Alaska Fisheries Science Center conducts annual bottom trawl surveys to monitor the condition of the demersal fish and crab stocks of the eastern Bering Sea continental shelf. -
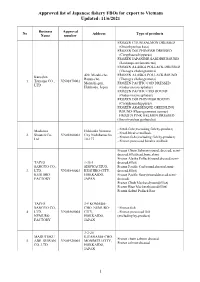
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

Strategic Science Plan Appendices
Aleutian and Bering Sea Islands Landscape Conservation Cooperative Strategic Science Plan Appendix B Appendix B. Climate Variability and Change. The resilience of many ecosystems is likely to be exceeded by an expected increase of 1.5 to 2.5°C in global average temperature over the 21st century. Associated disturbances affecting the frequency and intensity of storms, species range shifts and major trophic changes will likely combine with other threats. The overexploitation of resources, land-use change, pollution and fragmentation of natural systems are expected to profoundly affect all regions of the planet. The Intergovernmental Panel on Climate Change (IPCC) defines four areas of special concern, including two relevant to ABSI: the Arctic, because of the impacts of high rates of projected warming on natural systems and human communities; and small islands where there is high exposure of population and infrastructure to projected climate change impacts. Changes in ocean and air temperature in the ABSI region have resulted in changes in sea ice extent and season, species shifts, changes in storm regimes and coastal erosion. Further changes are expected in ocean circulation, salinity, and sea level, which collectively are expected to further threaten resources and the resilience of human communities. Adding to the concern is the fact that the Bering Sea has historically been quite sensitive to climate variability, with abrupt but persistent shifts in nutrient cycling and species assemblages linked to climate. This regional variation further complicates evaluating the likely regional impacts of global climate change. Key Affected Resources and Ecosystem Services: Subsistence Culture, Commercial Fishing, Marine Mammals, Seabirds, Trophic Structure, Community Resilience, Cultural Resources. -

Studies on the Detection Methods for Seafood Noxious Substances (水産食品における有害物質の検出法に関する研究)
博士論文(要約) Studies on the detection methods for seafood noxious substances (水産食品における有害物質の検出法に関する研究) 張 虹 I Abstract Food safety issues such as allergy and poisoning are always an important problem all around the world in the past decade. The consumers, governments and producers give increased attention to the safety and quality of food products in today’s global marketplace, and detection technologies consequently become more important for ensuring food safety and security. The present study focuses on the detection of the food allergy of invertebrate major allergen, tropomyosin, and food poisoning of natural marine biotoxin, tetrodotoxin (TTX). Food allergy is a dysfunction of the immune system against the ingested food or food additives. It is commonly mediated by immunoglobulin E (IgE) antibodies bound to mast cells, leading to release of histamine and other chemicals, which produces the symptoms such as urticaria, diarrhea, asthma and shock in severe cases. Seafood allergy is common and major causes of food allergy in adult. During recent decades, seafood allergy is becoming a serious problem with increasing seafood consumption. Studies on food allergies demonstrated that tropomyosin is an invertebrate major allergen and shows cross-reaction among the given invertebrate species. Biotoxins are substances synthesized by living organisms that are harmful to humans. They can be produced by many species of organisms such as bacteria, fungi, vertebrates, or marine microorganisms. The gastrointestinal tract and the nervous system of human can be affected by the biotoxins. One of popular biotoxin in Asia is TTX. TTX is a powerful neurotoxin found in several species, most notably in puffer fish. -

Biological Characterization: an Overview of Bristol, Nushagak, and Kvichak Bays; Essential Fish Habitat, Processes, and Species Assemblages
AN ASSESSMENT OF POTENTIAL MINING IMPACTS ON SALMON ECOSYSTEMS OF BRISTOL BAY, ALASKA VOLUME 3—APPENDICES E-J Appendix F: Biological Characterization: Bristol Bay Marine Estuarine Processes, Fish and Marine Mammal Assemblages Biological Characterization: An Overview of Bristol, Nushagak, and Kvichak Bays; Essential Fish Habitat, Processes, and Species Assemblages December 2013 Prepared by National Marine Fisheries Service, Alaska Region National Marine Fisheries Service, Alaska Region ii PREFACE The Bristol Bay watershed supports abundant populations of all five species of Pacific salmon found in North America (sockeye, Chinook, chum, coho, and pink), including nearly half of the world’s commercial sockeye salmon harvest. This abundance results from and, in turn, contributes to the healthy condition of the watershed’s habitat. In addition to these fisheries resources, the Bristol Bay region has been found to contain extensive deposits of low-grade porphyry copper, gold, and molybdenum in the Nushagak and Kvichak River watersheds. Exploration of these deposits suggests that the region has the potential to become one of the largest mining developments in the world. The potential environmental impacts from large-scale mining activities in these salmon habitats raise concerns about the sustainability of these fisheries for Alaska Natives who maintain a salmon-based culture and a subsistence lifestyle. Nine federally recognized tribes in Bristol Bay along with other tribal organizations, groups, and individuals have petitioned the U.S. Environmental -

Distribution, Abundance, and Biology of Blue King and Korean Hair Crabs Around the Pribilof Islands
DISTRIBUTION, ABUNDANCE, AND BIOLOGY OF BLUE KING AND KOREAN HAIR CRABS AROUND THE PRIBILOF ISLANDS by David A. Armstrong, Janet L. Armstrong, Gregory Jensen, Raul Palacios, and George Williams School of Fisheries University of Washington Seattle Washington 98195 Final Report Outer Continental Shelf Environmental Assessment Program Research Unit 638 July 1987 ACKNOWLEDGMENTS This study was funded wholly by the Minerals Management Service, Department of the Interior, through an Interagency Agreement with the National Oceanic and Atmospheric Administration, Department of Commerce, as part of the Alaska Outer Continental Shelf Environmental Assessment Program (OCSEAP). We thank the captain and crew of the RV Miller Freeman for all their efforts during the three cruises in May and August 1983 and April 1984. Commander Michael Fleming suggested use of sidescan sonar on this project and we appreciate his interest and help throughout. Paul Dinnel and Rae Baxter provided valuable field assistance. Murray Hayes of the Northwest and Alaska Fisheries Center authorized use of computer time and program accessibility at the National Marine Fisheries Service, Seattle, Washington, which greatly facilitated data analyses. Carla Norwood typed this document, and Dr. Paul Dinnel was instrumental in completion of the final report. Lyman Thorsteinson, Paul Becker, and Laurie Jarvela of NOAA, Ocean Assessments Division, Anchorage, Alaska, provided editorial assistance. We especially thank Lyman Thorsteinson for his patience and understanding. 3 ABSTRACT A series of three research cruises in 1983/1984 were used to characterize nearshore distribution and abundance as well as population dynamics and general ecology of blue king crab (Paralithodes platypus) and Korean hair crab (Erimacrus isenbeckii). -

North Pacific Fishery Management Council Fishery Management Options for the Alaskan EEZ in the Chukchi and Beaufort Seas Of
North Pacific Fishery Management Council Fishery Management Options for the Alaskan EEZ in the Chukchi and Beaufort Seas of the Arctic Ocean – A Revised Discussion Paper Prepared by Bill Wilson April 2007 (Revised May 2007) Contents I. Introduction II. Options for Fishery Management A. Alternative 1 - Status quo B. Alternative 2 - Amend the existing Fishery Management Plans C. Alternative 3 - Adopt a new FMP for the Arctic III. Next Steps IV. References V. Appendix Figure 1 I. Introduction At its October 2006 meeting, the Council asked staff to prepare a discussion paper on options for management of fisheries in the Alaskan Exclusive Economic Zone (EEZ) waters of the Arctic Ocean. The Council is interested in exploring policy options, such as a Fishery Management Plan, to conserve marine resources and manage existing or potential future commercial fisheries in this region. The Council received that report at the December meeting, and tasked staff to further develop options for fishery management in the Arctic. Specifically, the Council’s motion was: For waters north of Bering Strait, the Council moves to develop an analysis that would include the following alternatives: 1. Status quo for those waters. 2. Amend the existing scallop FMP, the BSAI groundfish FMP, and the BSAI king and Tanner crab FMP to prohibit commercial fishing in the Chukchi Sea. 3. Adopt a new FMP for the waters north of Bering Strait for any species not covered by an FMP (including krill and other forage species) with the following sub options: a) Close all Federal waters to commercial fishing until such time as the Council develops a policy for opening the waters to select commercial fishing practices, or b) Close all Federal waters north of Bering Strait to commercial fishing for forage species, and all waters north of a line at Point Hope to commercial fishing for all species (see Figure 1 map in staff discussion paper). -

Characterization of Seafood Proteins Causing Allergic Diseases
5 Characterization of Seafood Proteins Causing Allergic Diseases Anas M. Abdel Rahman1, Robert J. Helleur2, Mohamed F. Jeebhay3 and Andreas L. Lopata4 1Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON, 2Department of Chemistry, Memorial University of Newfoundland, St. John’s, NL 3Centre for Occupational and Environmental Health Research, University of Cape Town 4School of Pharmacy and Molecular Science, James Cook University 1,2Canada 3South Africa 4Australia 1. Introduction Food allergy is increasing at a faster rate than any other allergic disorder (Gupta et al., 2007). In the last few decades, a large movement toward healthier eating makes seafood one of the major foods consumed worldwide (Wild & Lehrer, 2005). Consequently, the international trade of seafood has been growing rapidly, which reflects the popularity and frequency of consumption worldwide. The United States has become the third largest consumer of seafood in the world, with 1.86 billion kg of crustaceans in 2007 (6.04 kg/capita/year)(Food and Agriculture Organisation, 2007). Since seafood ingestion can cause severe acute hypersensitivity reactions and is recognized as one of the most common food allergies, the increased production and consumption of seafood has resulted in more frequent health problems (Lopata & Lehrer, 2009; Lopata et al., 2010). Exposure to seafood can cause a variety of health problems, including gastrointestinal disorders, urticaria, immunoglobulin E (IgE)-mediated asthma and anaphylaxis (Bang et al., 2005; Lopata & Lehrer, 2009; Malo & Cartier, 1993; Sicherer et al., 2004; Wild & Lehrer, 2005). A true allergy is known as type-one hypersensitivity that activates the human mast cells, a type of white blood cells, producing an IgE response as seen in Fig. -

Morphometric Approaches Reveal Sexual Differences in the Carapace Shape of the Horsehair Crab, Erimacrus Isenbeckii (Brandt, 1848)
水生動物 第 2020 巻 令和 2 年 3 月 Morphometric approaches reveal sexual differences in the carapace shape of the horsehair crab, Erimacrus isenbeckii (Brandt, 1848) Kenji Toyota1,2*, Kentaro Izumi3, Takashi Ichikawa4, Tsuyoshi Ohira1, Ken Takeuchi2,5 1Department of Biological Sciences, Faculty of Science, Kanagawa University, 2946 Tsuchiya, Hiratsuka, Kanagawa, 259-1293, Japan. 2Department of Biological Science and Technology, Faculty of Industrial Science and Technology, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan. 3Faculty & Graduate School of Education, Chiba University, 1-33 Yoyoi-cho, Inage-ku, Chiba-shi, Chiba 263-8522, Japan. 4Tokyo University of Agriculture, 196 Yasaka, Abashiri-shi, Hokkaido, 099-2493, Japan. 5Department of Biological Science and Technology, Faculty of Industrial Science and Technology, Tokyo University of Science, 102-1 Tomino, Oshamambe-cho, Yamakoshi-gun, Hokkaido, 049-3514, Japan *Corresponding author; e-mail: [email protected]. Abstract Animal shape has become sophisticated through evolution or phenotypic plasticity to adapt and survive changing environmental conditions. Sexual differences, which are sex-specific characteristics, form an important research topic that has also long fascinated biologists. Morphometrics is a traditional approach that enables the quantitative description and interpretation of variation in shape based on morphological features. In this study, we attempted to apply two morphometrics approaches (landmark-based or measurement-based) to identify sexual dimorphic traits in the carapace of the horsehair crab, Erimacrus isenbeckii (Brandt, 1848). Both approaches revealed that its carapace showed apparent sexual dimorphism, enabling the discrimination of the male and female carapace with more than 90 % accuracy. Moreover, the landmark-based approach found that carapace shape showed fluctuating asymmetry as well as directional asymmetry in both males and females. -

2002 Aleutian Islands Bottom Trawl Survey
APPENDIX A Description of Sampling Gear Table A-1 is a summarizes specifications of the Poly-Nor’Eastern trawl and Figure A-1 is a schematic diagram of the trawl and accessories used during the 2002 Aleutian Islands bottom trawl survey. 225 226 Appendix Table A-1.--Specifications of the trawl, otter doors, and accessory gear used during the 2002 Aleutian Islands bottom trawl survey. Equipment Specifications Netting - Polyethylene, 12.7 cm (5 in), 4 & 5 millimeter (mm) twine. Headrope - 27.2 m (89 ft-1 in), 1.3 cm (1/2 in) 6 x 19 gauge galvanized wire rope, wrapped with 1 cm (3/8 in) 3-strand polypropylene rope. Footrope - 24.9 m (81 ft-7 in), 1 cm (3/8 in) 6 x 19 gauge galvanized wire rope wrapped with polypropylene rope. Fishing line - 24.3 m (79 ft-7 in), shot-peened long-link chain. Safe working load 5.1 metric ton (t). Roller gear - 24.2 m (79 ft-6 in), 1.9 cm (3/4 in) diameter, 6 x 19 gauge galvanized wire rope strung through 10.2 cm (4 in) diameter rubber disks, 16 rubber wing bobbins 35.6 cm (14 in) in diameter and 34 galvanized rings hung with 30.5 cm (12 in) long dropper chain. Breastlines - 1 cm (3/8 in) 6 x 19 gauge galvanized wire rope wrapped with 1 cm polypropylene. Top corner 5.9 m (19 ft-6 in); bottom corner 2.6 m (8 ft-8 in); top side panel 5.9 m; bottom side panel 9.3 m (30 ft-6 in). -

50 CFR Ch. VI (10–1–09 Edition) Pt. 680, Table 2
Pt. 680, Table 2 50 CFR Ch. VI (10–1–09 Edition) TABLE 2 TO PART 680—CRAB SPECIES CODE Species code Common name Scientific name 900 ........................ Box ....................................................................... Lopholithodes mandtii. 910 ........................ Dungeness ........................................................... Cancer magister. 921 ........................ Red king crab ....................................................... Paralithodes camtshaticus. 922 ........................ Blue king crab ...................................................... Paralithodes platypus. 923 ........................ Golden (brown) king crab .................................... Lithodes aequispinus. 924 ........................ Scarlet king crab .................................................. Lithodes couesi. 931 ........................ Tanner crab .......................................................... Chionoecetes bairdi. 932 ........................ Snow crab ............................................................ Chionoecetes opilio. 933 ........................ Grooved Tanner crab ........................................... Chionoecetes tanneri. 934 ........................ Triangle Tanner crab ........................................... Chionoecetes angulatus. 940 ........................ Korean horsehair crab ......................................... Erimacrus isenbeckii. 951 ........................ Multispinus crab ................................................... Paralomis multispinus. 953