Characterization of Seafood Proteins Causing Allergic Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shellfish Allergy - an Asia-Pacific Perspective
Review article Shellfish allergy - an Asia-Pacific perspective 1 1 1 2 Alison Joanne Lee, Irvin Gerez, Lynette Pei-Chi Shek and Bee Wah Lee Summary Conclusion: Shellfish allergy is common in the Background and Objective: Shellfish forms a Asia Pacific. More research including food common food source in the Asia-Pacific and is challenge-proven subjects are required to also growing in the West. This review aims to establish the true prevalence, as well as to summarize the current literature on the understand clinical cross reactivity and epidemiology and research on shellfish allergy variations in clinical features. (Asian Pac J Allergy with particular focus on studies emerging from Immunol 2012;30:3-10) the Asia-Pacific region. Key words: Shellfish allergy, Prawn allergy, Shrimp Data Sources: A PubMed search using search allergy, Food allergy, Anaphylaxis, Tropomyosin, strategies “Shellfish AND Allergy”, “Shellfish Allergy Asia”, and “Shellfish AND anaphylaxis” Allergens, Asia was made. In all, 244 articles written in English were reviewed. Introduction Shellfish, which include crustaceans and Results: Shellfish allergy in the Asia-Pacific molluscs, is one of the most common causes of food ranks among the highest in the world and is the allergy in the world in both adults and children, and most common cause of food-induced anaphylaxis. it has been demonstrated to be one of the top Shellfish are classified into molluscs and ranking causes of food allergy in children in the arthropods. Of the arthropods, the crustaceans Asia-Pacific.1-3 In addition, shellfish allergy usually in particular Penaeid prawns are the most persists, is one of the leading causes of food-induced common cause of allergy and are therefore most anaphylaxis, and has been implicated as the most extensively studied. -

History of Alaska Red King Crab, Paralithodes Camtschaticus, Bottom Trawl Surveys, 1940–61
History of Alaska Red King Crab, Paralithodes camtschaticus, Bottom Trawl Surveys, 1940–61 MARK ZIMMERMANN, C. BRAXTON DEW, and BEVERLY A. MALLEY Introduction As early as the 1930’s, the Alaska ≥135 mm) was recorded in 1980. Then, red king crab resource was a heav- in 1981, the Bristol Bay red king crab The U.S. government was integrally ily exploited species, with significant population abruptly collapsed in one involved with the development of Alas- foreign commercial harvests occurring of the more precipitous declines in the ka’s red king crab, Paralithodes camts- well before the first U.S. (1940–41) ex- history of U.S. fisheries management. chaticus, fishing industry (Blackford, ploratory bottom trawl survey (Schmitt, In the opinion of Alaska crab manag- 1979). In 1940 Congress appropriated 1940; FWS, 1942). During the 1930’s, ers and modelers (Otto, 1986; Zheng funds for surveys of Alaska’s fishery Japan and Russia together took 9–14 and Kruse, 2002; NPFMC1), the crash resources (Schmitt, 1940; FWS, 1942). million kg of king crab per year from of the Bristol Bay red king crab stock Based on the U.S. Fish and Wildlife the southeast Bering Sea, within the vast was a natural phenomenon unrelated to Service’s (FWS) exploratory work for area referred to as Bristol Bay. commercial fishing. red king crab during the 1940’s, skepti- In 1946, U.S. fishermen began com- Considering the history of the Bristol cal commercial trawlers expected the mercial king crab fishing in these same Bay red king crab commercial fishery bulk of their profits to come from bot- waters, and by 1963 the United States and the unresolved issues surrounding tomfish. -
What You Need to Know
WhatWhat YouYou NeedNeed ToTo KnowKnow 11 inin 1313 childrenchildren That’sThat’s roughlyroughly inin thethe U.S.U.S. hashas 66 millionmillion aa foodfood allergy...allergy... children.children. That'sThat's aboutabout 22 kidskids inin everyevery classroom.classroom. MoreMore thanthan 15%15% ofof schoolschool agedaged childrenchildren withwith foodfood allergiesallergies havehave hadhad aa reactionreaction inin school.school. A food allergy occurs when Food allergy is an IgE-mediated immune the immune system targets a reaction and is not the same as a food food protein and sets off a intolerance or sensitivity. It occurs quickly reaction throughout the body. and can be life-threatening. IgE, or Immunoglobulin E, are antibodies produced by the immune system. IgE antibodies fight allergenic food by releasing chemicals like histamine that trigger symptoms of an allergic reaction. ANTIBODY HISTAMINE Mild Symptoms Severe Symptoms Redness around Swelling of lips, Difficulty Hives mouth or eyes tongue and/or throat swallowing Shortness of Low blood Itchy mouth, Stomach pain breath, wheezing pressure nose or ears or cramps Loss of Chest pain consciousness Nausea or Sneezing Repetitive coughing vomiting AccordingAccording toto thethe CDC,CDC, foodfood allergiesallergies resultresult inin moremore thanthan 300,000300,000 doctordoctor visitsvisits eacheach yearyear amongamong childrenchildren underunder thethe ageage ofof 18.18. Risk Factors Family history Children are at Having asthma or an of asthma or greater risk allergic condition such allergies than adults as hay fever or eczema WhatWhat CanCan YouYou BeBe AllergicAllergic To?To? Up to 4,145,700 people in the U.S. are PEANUTSPEANUTS allergic to peanuts. That’s more than the entire country population 25-40% of Panama! of people who are allergic to peanuts also have reactions to at least one tree nut. -

SAMPLE REPORT - STANDARD 150 We Hope That the Test Result & Report Will Give You Satisfactory Information and That It Enables You, to Make Informed Decisions
Independent Alternative Allergy Specialist Tripenhad, Tripenhad Road, Ferryside, SA17 5RS, Wales/UK 0345 094 3298 | [email protected] | www.allergylink.co.uk Combination Allergy & Intolerance Test Food Intolerance & Substance Sensitivity - Standard 150 - Ref No: AL#438 Name: SAMPLE Date: 15 January 2020 SSAAMMPPLLEE RREEPPOORRTT -- SSTTAANNDDAARRDD 115500 ©©CCooppyyrriigghhtt –– 22000044--22002200 AAlllleerrggyy LLiinnkk Updated: 15.01.2020 www.allergylink.co.uk ©2004-2020 Allergy Link SAMPLE REPORT - STANDARD 150 We hope that the Test Result & Report will give you satisfactory information and that it enables you, to make informed decisions. Content & Reference: Part 1: Test -Table 3 Low Stomach Acid / Enzyme deficiency 18 Part 2: About your Report 4-5 Other possible cause of digestive problems 19-20 About Allergies and Intolerances 5-7 Non-Food Items & Substances Part 3: Allergen’s and Reactions Explained 8 Pet Allergies, House dust mite & Pollen 20-21 Dairy / Lactose 8 Environmental toxins 21 Eggs & Chicken 9 Pesticides and Herbicides 21 Fish, Shellfish, Glucosamine 9 Fluoride (now classified as a neurotoxin) 22 Coffee, Caffeine & Cocoa / Corn 10 Formaldehyde, Chlorine 22 Gluten / Wheat 10 Toiletries / Preservatives MI / MCI 23 Yeast & Moulds 11 Perfume / Fragrance 23 Alcohol - beer, wine 11 Detergents & Fabric conditioners 23-24 Fruit , Citrus fruit 12 Aluminium, Nickel, Teflon, Latex 24 Fructose / FODMAPs | Cellery 12 Vegetables | Nightshades, Tomato 13 Part 4: Beneficial Supplementing of Vitamins & Minerals Soya , bean, products -

Welcoming Guests with Food Allergies
Welcoming Guests With Food Allergies A comprehensive program for training staff to safely prepare and serve food to guests who have food allergies The Food Allergy & Anaphylaxis Network 11781 Lee Jackson Hwy., Suite 160 Fairfax, VA 22033 (800) 929-4040 www.foodallergy.org Produced and distributed by the Food Allergy & Anaphylaxis Network (FAAN). FAAN is a nonprofit organization established to raise public awareness, to provide advocacy and education, and to advance research on behalf of all those affected by food allergies and anaphylaxis. All donations are tax-deductible. © 2001. Updated 2010, the Food Allergy & Anaphylaxis Network. All Rights Reserved. ISBN 1-882541-21-9 FAAN grants permission to photocopy this document for limited internal use. This consent does not extend to other kinds of copying, such as copying for general distribution (excluding the materials in the Appendix, which may be customized, reproduced, and distributed for and by the establishment), for advertising or promotional purposes, for creating new collective works, or for resale. For information, contact FAAN, 11781 Lee Jackson Hwy., Suite 160, Fairfax, VA 22033, www.foodallergy.org. Disclaimer This guide was designed to provide a guideline for restaurant and food service employees. FAAN and its collaborators disclaim any responsibility for any adverse effects resulting from the information presented in this guide. FAAN does not warrant or guarantee that following the procedures outlined in this guide will eliminate or prevent allergic reactions. The food service facility should not rely on the information contained herein as its sole source of information to prevent allergic reactions. The food service facility should make sure that it complies with all local, state, and federal requirements relating to the safe handling of food and other consumable items, in addition to following safe food-handling procedures to prevent food contamination. -

Allergens Are Not Detected in the Bronchoalveolar Lavage Fluid Of
Practitioner's Corner 148 4. Spierings NM, Natkunarajah J, Bansal A, Ostlere L. Should we be prescribing isotretinoin to patients with peanut allergies? Allergens Are Not Detected in the Bronchoalveolar Clin Exp Dermatol. 2015;40:824-5. Lavage Fluid of Patients Undergoing Fiberoptic 5. Kukkonen AK, Pelkonen AS, Makinen-Kiljunen S, Voutilainen Bronchoscopy H, Makela MJ. Ara h 2 and Ara 6 are the best predictors of severe peanut allergy: a double-blind placebo-controlled Rueda M1, López-Matas MA2, Agustí C3, Lucena C3, Carnés J2, study. Allergy. 2015;70:1239-45. Valero A3,4 6. Holzhauser T, Wackermann O, Ballmer-Weber BK, et al. 1Allergology Service, Hospital Quirónsalud, Barcelona, Spain Soybean (Glycine max) allergy in Europe: Gly m 5 (beta- 2R&D Department, Laboratorios LETI S.L.U., Tres Cantos, conglycinin) and Gly m 6 (glycinin) are potential diagnostic Madrid, Spain markers for severe allergic reactions to soy. J Allergy Clin 3Neumology and Allergy Service, Hospital Clinic, Barcelona, Immunol. 2009;123:452-8. Spain 7. Sicherer SH, Sampson HA, Burks AW. Peanut and soy allergy: 4Institut d’Investigacions Biomèdiques August Pi i Sunyer a clinical and therapeutic dilemma. Allergy. 2000;55:515-21. (IDIBAPS), Centro de Investigaciones Biomédicas en Red de 8. Awazuhara H, Kawai H, Baba M, Matsui T, Komiyama A. Enfermedades Respiratorias (CIBERES), Barcelona, Spain Antigenicity of the proteins in soy lecithin and soy oil in soybean allergy. Clin Exp Allergy. 1998;28:1559-64. J Investig Allergol Clin Immunol 2019; Vol. 29(2): 148-150 9. Alden K, Chowdhury MMU, Williams PE, Kalavala M. Protocol doi: 10.18176/jiaci.0353 for investigation of possible soya allergy in patients being considered for treatment with isotretinoin or alitretinoin. -

2005 Bottom Trawl Survey of the Eastern Bering Sea Continental Shelf
Alaska Fisheries Science Center National Marine Fisheries Service U.S DEPARTMENT OF COMMERCE AFSC PROCESSED REPORT 2007-01 2005 Bottom Trawl Survey of the Eastern Bering Sea Continental Shelf January 2007 This report does not constitute a publication and is for information only. All data herein are to be considered provisional. This document should be cited as follows: Lauth, R, and E. Acuna (compilers). 2007. 2005 bottom trawl survey of the eastern Bering Sea continental shelf. AFSC Processed Rep. 2007-1, 164 p. Alaska Fish. Sci. Cent., NOAA, Natl. Mar, Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. Notice to Users of this Document This document is being made available in .PDF format for the convenience of users; however, the accuracy and correctness of the document can only be certified as was presented in the original hard copy format. 2005 BOTTOM TRAWL SURVEY OF THE EASTERN BERING SEA CONTINENTAL SHELF Compilers Robert Lauth Erika Acuna Bering Sea Subtask Erika Acuna Lyle Britt Jason Conner Gerald R. Hoff Stan Kotwicki Robert Lauth Gary Mundell Daniel Nichol Duane Stevenson Ken Weinberg Resource Assessment and Conservation Engineering Division Alaska Fisheries Science Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 7600 Sand Point Way N.E. Seattle, WA 98115-6349 January 2007 ABSTRACT The Resource Assessment and Conservation Engineering Division of the Alaska Fisheries Science Center conducts annual bottom trawl surveys to monitor the condition of the demersal fish and crab stocks of the eastern Bering Sea continental shelf. -
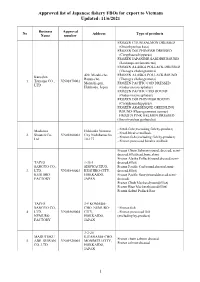
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

Edible Insects As a Source of Food Allergens Lee Palmer University of Nebraska-Lincoln, [email protected]
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations, Theses, & Student Research in Food Food Science and Technology Department Science and Technology 12-2016 Edible Insects as a Source of Food Allergens Lee Palmer University of Nebraska-Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/foodscidiss Part of the Food Chemistry Commons, and the Other Food Science Commons Palmer, Lee, "Edible Insects as a Source of Food Allergens" (2016). Dissertations, Theses, & Student Research in Food Science and Technology. 78. http://digitalcommons.unl.edu/foodscidiss/78 This Article is brought to you for free and open access by the Food Science and Technology Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations, Theses, & Student Research in Food Science and Technology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. EDIBLE INSECTS AS A SOURCE OF FOOD ALLERGENS by Lee Palmer A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Food Science and Technology Under the Supervision of Professors Philip E. Johnson and Michael G. Zeece Lincoln, Nebraska December, 2016 EDIBLE INSECTS AS A SOURCE OF FOOD ALLERGENS Lee Palmer, M.S. University of Nebraska, 2016 Advisors: Philip E. Johnson and Michael G. Zeece Increasing global population increasingly limited by resources has spurred interest in novel food sources. Insects may be an alternative food source in the near future, but consideration of insects as a food requires scrutiny due to risk of allergens. -

Strategic Science Plan Appendices
Aleutian and Bering Sea Islands Landscape Conservation Cooperative Strategic Science Plan Appendix B Appendix B. Climate Variability and Change. The resilience of many ecosystems is likely to be exceeded by an expected increase of 1.5 to 2.5°C in global average temperature over the 21st century. Associated disturbances affecting the frequency and intensity of storms, species range shifts and major trophic changes will likely combine with other threats. The overexploitation of resources, land-use change, pollution and fragmentation of natural systems are expected to profoundly affect all regions of the planet. The Intergovernmental Panel on Climate Change (IPCC) defines four areas of special concern, including two relevant to ABSI: the Arctic, because of the impacts of high rates of projected warming on natural systems and human communities; and small islands where there is high exposure of population and infrastructure to projected climate change impacts. Changes in ocean and air temperature in the ABSI region have resulted in changes in sea ice extent and season, species shifts, changes in storm regimes and coastal erosion. Further changes are expected in ocean circulation, salinity, and sea level, which collectively are expected to further threaten resources and the resilience of human communities. Adding to the concern is the fact that the Bering Sea has historically been quite sensitive to climate variability, with abrupt but persistent shifts in nutrient cycling and species assemblages linked to climate. This regional variation further complicates evaluating the likely regional impacts of global climate change. Key Affected Resources and Ecosystem Services: Subsistence Culture, Commercial Fishing, Marine Mammals, Seabirds, Trophic Structure, Community Resilience, Cultural Resources. -

Shellfish Or Fish Allergy E-Mail: [email protected] Letter: NUH NHS Trust, C/O PALS, Freepost NEA 14614, Information for Parents Nottingham NG7 1BR
Feedback We appreciate and encourage feedback. If you need advice or are concerned about any aspect of care or treatment please speak to a member of staff or contact the Patient Advice and Liaison Service (PALS): Freephone: 0800 183 0204 From a mobile or abroad: 0115 924 9924 ext 65412 or 62301 Shellfish or fish allergy E-mail: [email protected] Letter: NUH NHS Trust, c/o PALS, Freepost NEA 14614, Information for parents Nottingham NG7 1BR www.nuh.nhs.uk This information can be provided in different languages and formats. For more information please contact the: The Trust endeavours to ensure that the information given here Children’s Clinic is accurate and impartial. Tel: 0115 9249924 ext. 62661/64008 This leaflet has been adapted from the leaflet produced for the adult immunology department. Original leaflet produced by Lisa Slater. Debra Forster, Nottingham Children’s Hospital © October 2015. All rights reserved. Nottingham University Hospitals NHS Trust. Review October 2017. Ref: 0932/v3/1015/AS. Public information Shellfish or Fish Allergy Additional support Allergy to fish – such as cod and other white fish is likely to be Contact details life-long and may begin in childhood. Debra Forster, Children’s Respiratory & Allergy Nurse Children’s Clinic South Adverse reactions to shellfish are not usually seen until the B Floor, Nottingham Children’s Hospital teenage years or adulthood. This may be because shellfish is Queen’s Medical Centre not often a part of the diet of younger children. Nottingham NG7 2UH Symptoms Tel: 0115 9249924 ext 62501 Mild symptoms may include: The Anaphylaxis Campaign is a national charity that can Tummy pain and vomiting provide support and information. -

Shellfish/Crustacean Oral Allergy Syndrome Among National Service Pre-Enlistees in Singapore
Asia Pac Allergy. 2018 Apr;8(2):e18 https://doi.org/10.5415/apallergy.2018.8.e18 pISSN 2233-8276·eISSN 2233-8268 Original Article Shellfish/crustacean oral allergy syndrome among national service pre-enlistees in Singapore Bernard Yu-Hor Thong 1,*, Shalini Arulanandam2, Sze-Chin Tan1, Teck-Choon Tan1, Grace Yin-Lai Chan1, Justina Wei-Lyn Tan1, Mark Chong-Wei Yeow2, Chwee-Ying Tang1, Jinfeng Hou1, and Khai-Pang Leong1 1Department of Rheumatology, Allergy and Immunology, Tan Tock Seng Hospital, Singapore 308433 2Medical Classification Centre, Ministry of Defence, Singapore 109680 Received: Jan 21, 2018 ABSTRACT Accepted: Apr 8, 2018 Background: *Correspondence to All Singaporean males undergo medical screening prior to compulsory military Bernard Yu-Hor Thong service. A history of possible food allergy may require referral to a specialist Allergy clinic to Department of Rheumatology, Allergy and ensure that special dietary needs can be taken into account during field training and deployment. Immunology, Tan Tock Seng Hospital, 11 Jalan Objective: To study the pattern of food allergy among pre-enlistees who were referred to a Tan Tock Seng, Singapore 308433. specialist allergy clinic to work up suspected food allergy. Tel: +65-63577822 Methods: Retrospective study of all pre-enlistees registered in the Clinical Immunology/ Fax: +65-63572686 E-mail: [email protected] Allergy New Case Registry referred to the Allergy Clinic from 1 August 2015 to 31 May 2016 for suspected food allergy. Copyright © 2018. Asia Pacific Association of Results: One hundred twenty pre-enlistees reporting food allergy symptoms other than rash Allergy, Asthma and Clinical Immunology. alone were referred to the Allergy Clinic during the study period.