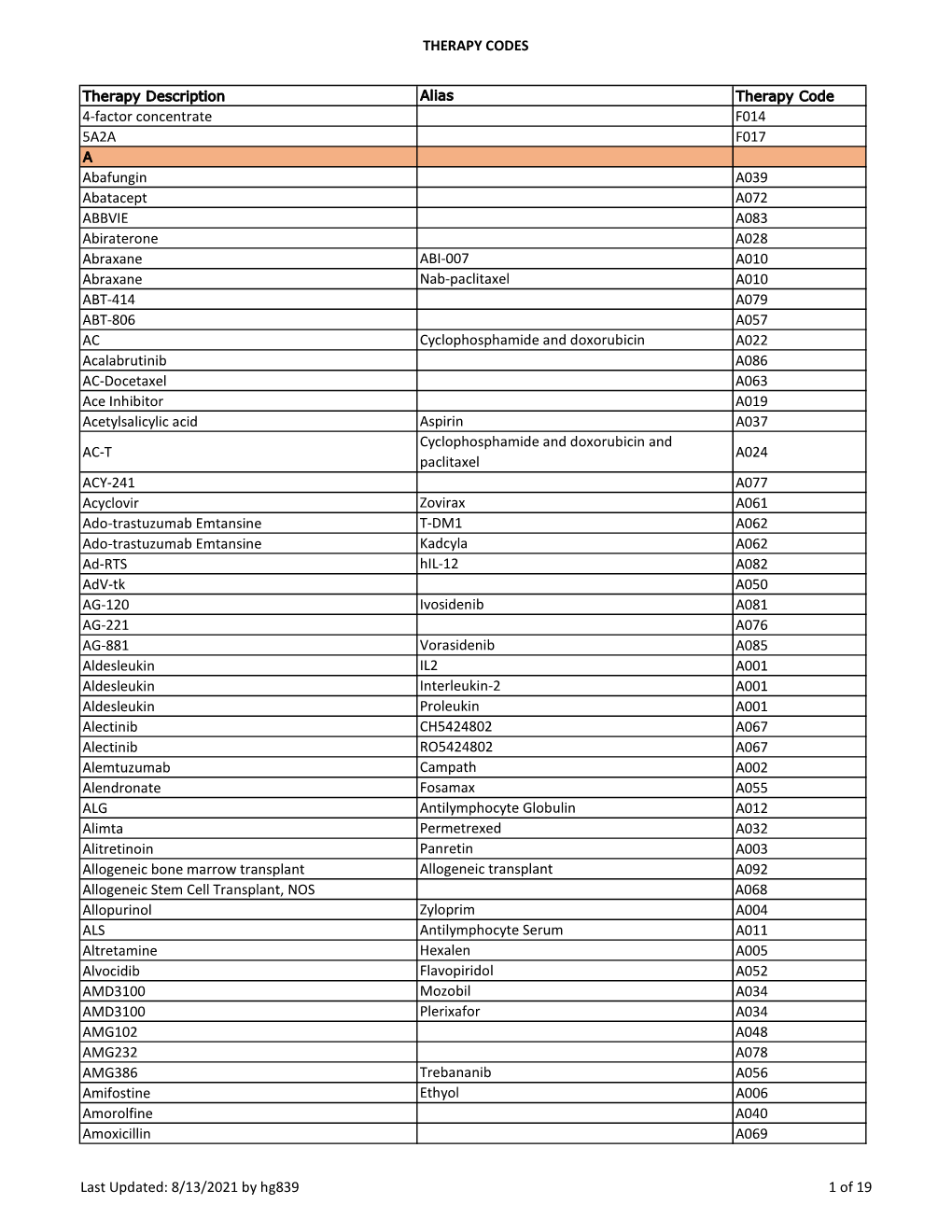
THERAPY CODES 4-Factor Concentrate F014 5A2A F017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HER2 Inhibition in Gastro-Oesophageal Cancer: a Review Drawing on Lessons Learned from Breast Cancer
Submit a Manuscript: http://www.f6publishing.com World J Gastrointest Oncol 2018 July 15; 10(7): 159-171 DOI: 10.4251/wjgo.v10.i7.159 ISSN 1948-5204 (online) REVIEW HER2 inhibition in gastro-oesophageal cancer: A review drawing on lessons learned from breast cancer Hazel Lote, Nicola Valeri, Ian Chau Hazel Lote, Nicola Valeri, Centre for Molecular Pathology, Accepted: May 30, 2018 Institute of Cancer Research, Sutton SM2 5NG, United Kingdom Article in press: May 30, 2018 Published online: July 15, 2018 Hazel Lote, Nicola Valeri, Ian Chau, Department of Medicine, Royal Marsden Hospital, Sutton SM2 5PT, United Kingdom ORCID number: Hazel Lote (0000-0003-1172-0372); Nicola Valeri (0000-0002-5426-5683); Ian Chau (0000-0003-0286-8703). Abstract Human epidermal growth factor receptor 2 (HER2)- Author contributions: Lote H wrote the original manuscript and revised it following peer review comments; Valeri N reviewed inhibition is an important therapeutic strategy in HER2- the manuscript; Chau I reviewed and contributed to the content of amplified gastro-oesophageal cancer (GOC). A significant the manuscript. proportion of GOC patients display HER2 amplification, yet HER2 inhibition in these patients has not displayed Supported by National Health Service funding to the National the success seen in HER2 amplified breast cancer. Mu- Institute for Health Research Biomedical Research Centre at ch of the current evidence surrounding HER2 has been the Royal Marsden NHS Foundation Trust and The Institute of obtained from studies in breast cancer, and we are only re- Cancer Research, No. A62, No. A100, No. A101 and No. A159; Cancer Research UK funding, No. -

Universidade Federal Da Paraíba Centro De Ciências Da Saúde
Universidade Federal da Paraíba Centro de Ciências da Saúde Programa de Pós-Graduação em Produtos Naturais e Sintéticos Bioativos Wylly Araújo de Oliveira Atividade do óleo essencial de Cymbopogon winterianus Jowitt ex Bor contra Candida albicans , Aspergillus flavus e Aspergillus fumigatus João Pessoa-PB 2011 Wylly Araújo de Oliveira Atividade do óleo essencial de Cymbopogon winterianus Jowitt ex Bor contra Candida albicans , Aspergillus flavus e Aspergillus fumigatus Tese de doutorado apresentada ao Programa de Pós-Graduação em Produtos Naturais e Sintéticos Bioativos, Centro de Ciências da Saúde, Universidade Federal da Paraíba, em cumprimento aos requisitos necessários para a obtenção do título de Doutor em Produtos Naturais e Sintéticos Bioativos, área de concentração: farmacologia Orientadora: Prof.ª Dr.ª Edeltrudes de Oliveira Lima João Pessoa-PB 2011 Wylly Araújo de Oliveira Atividade do óleo essencial de Cymbopogon winterianus Jowitt ex Bor contra Candida albicans, Aspergillus flavus e Aspergillus fumigatus Tese de Doutorado aprovada em 22/06/2011 Banca examinadora ________________________________________________ Prof.ª Dr.ª Edeltrudes de Oliveira Lima Orientadora/UFPB _________________________________________________ Prof.ª Dr.ª Hilzeth de Luna Freire Pessôa - UFPB _________________________________________________ Prof. Dr. José Pinto de Siqueira Júnior - UFPB __________________________________________________ Prof.ª Dr.ª Margareth de Fátima Formiga Melo Diniz - UFPB __________________________________________________ Prof. Dr. Thompson Lopes de Oliveira - UFPB Dedicatória Com amor, dedico este trabalho à minha família: a meu pai, Francisco Claro de Oliveira; a minha mãe, Maria Araújo Filha; e a meus irmãos Kylly Araújo de Oliveira e Welly Araújo de Oliveira. Sem o apoio deles, nada disso teria sido possível. Apesar da ausência, eles sempre estiveram no meu coração. -

Cancer Subtype Identification Using Somatic Mutation Data
www.nature.com/bjc ARTICLE Genetics and Genomics Cancer subtype identification using somatic mutation data Marieke Lydia Kuijjer 1,2, Joseph Nathaniel Paulson1,2,3, Peter Salzman4, Wei Ding5 and John Quackenbush1,2,6 BACKGROUND: With the onset of next-generation sequencing technologies, we have made great progress in identifying recurrent mutational drivers of cancer. As cancer tissues are now frequently screened for specific sets of mutations, a large amount of samples has become available for analysis. Classification of patients with similar mutation profiles may help identifying subgroups of patients who might benefit from specific types of treatment. However, classification based on somatic mutations is challenging due to the sparseness and heterogeneity of the data. METHODS: Here we describe a new method to de-sparsify somatic mutation data using biological pathways. We applied this method to 23 cancer types from The Cancer Genome Atlas, including samples from 5805 primary tumours. RESULTS: We show that, for most cancer types, de-sparsified mutation data associate with phenotypic data. We identify poor prognostic subtypes in three cancer types, which are associated with mutations in signal transduction pathways for which targeted treatment options are available. We identify subtype–drug associations for 14 additional subtypes. Finally, we perform a pan-cancer subtyping analysis and identify nine pan-cancer subtypes, which associate with mutations in four overarching sets of biological pathways. CONCLUSIONS: This study is an important step toward understanding mutational patterns in cancer. British Journal of Cancer (2018) 118:1492–1501; https://doi.org/10.1038/s41416-018-0109-7 INTRODUCTION However, subtype classification using somatic mutations in Cancer is a heterogeneous disease that can develop in different cancer is challenging, mainly because the data are very sparse: tissues and cell types. -

WO 2018/223101 Al 06 December 2018 (06.12.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/223101 Al 06 December 2018 (06.12.2018) W !P O PCT (51) International Patent Classification: (71) Applicant: JUNO THERAPEUTICS, INC. [US/US]; 400 A 61K 35/1 7 (20 15.0 1) A 61P 35/00 (2006 .0 1) Dexter Ave. N., Suite 1200, Seattle, WA 98109 (US). (21) International Application Number: (72) Inventor: ALBERTSON, Tina; 400 Dexter Ave. N., Suite PCT/US2018/035755 1200, Seattle, WA 98109 (US). (22) International Filing Date: (74) Agent: AHN, Sejin et al; Morrison & Foerster LLP, 1253 1 0 1 June 2018 (01 .06.2018) High Bluff Drive, Suite 100, San Diego, CA 92130-2040 (US). (25) Filing Language: English (81) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of national protection available): AE, AG, AL, AM, (30) Priority Data: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, 62/5 14,774 02 June 2017 (02.06.2017) US CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, 62/5 15,530 05 June 2017 (05.06.2017) US DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, 62/521,366 16 June 2017 (16.06.2017) u s HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, 62/527,000 29 June 2017 (29.06.2017) u s KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, 62/549,938 24 August 2017 (24.08.2017) u s MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 62/580,425 0 1 November 2017 (01 .11.2017) u s OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 62/593,871 0 1 December 2017 (01 .12.2017) u s SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 62/596,764 08 December 2017 (08.12.2017) u s TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Suppression of Signal Transducer and Activator of Transcription 3 Activation by Butein Inhibits Growth of Human Hepatocellular Carcinoma in Vivo
Author Manuscript Published OnlineFirst on December 3, 2010; DOI: 10.1158/1078-0432.CCR-10-1123 AuthorPublished manuscripts OnlineFirst have been on peer December reviewed and 3, accepted 2010 as for 10.1158/1078-0432.CCR-10-1123 publication but have not yet been edited. Suppression of Signal Transducer and Activator of Transcription 3 Activation by Butein Inhibits Growth of Human Hepatocellular Carcinoma in vivo Peramaiyan Rajendran1, Tina H. Ong2, Luxi Chen1,3, Feng Li1, Muthu K Shanmugam1, Shireen Vali4, Taher Abbasi4, Shweta Kapoor4, Ashish Sharma4, Alan Prem Kumar1,3, Kam M. Hui2,5 Gautam Sethi1,5 1Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, 2Division of Cellular and Molecular Research, Humphrey Oei Institute of Cancer Research National Cancer Centre, Singapore 169610, 3Cancer Science Institute of Singapore, National University of Singapore, and 4Cellworks Group Inc., California 95070; 4Cellworks Research India Pvt. Ltd, Bangalore 560066, India. Running title: Butein inhibits STAT3 signaling in vitro and in vivo in HCC. 5To whom correspondence should be addressed: 1. Dr. Gautam Sethi, Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Phone: +65-65163267; Fax: +65- 68737690; Email: [email protected] Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Copyright © 2010 American Association for Cancer Research Downloaded from clincancerres.aacrjournals.org on September 27, 2021. © 2010 American Association for Cancer Research. Author Manuscript Published OnlineFirst on December 3, 2010; DOI: 10.1158/1078-0432.CCR-10-1123 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -

Or Ramucirumab (IMC-1121B) Plus Mitoxantrone and Prednisone in Men with Metastatic Docetaxel-Pretreated Castration-Resistant Prostate Cancer
European Journal of Cancer (2015) 51, 1714– 1724 Available at www.sciencedirect.com ScienceDirect journal homepage: www.ejcancer.com A randomised non-comparative phase II trial of cixutumumab (IMC-A12) or ramucirumab (IMC-1121B) plus mitoxantrone and prednisone in men with metastatic docetaxel-pretreated castration-resistant prostate cancer Maha Hussain a,1,⇑, Dana Rathkopf b,1, Glenn Liu c,1, Andrew Armstrong d,1, Wm. Kevin Kelly e, Anna Ferrari f, John Hainsworth g, Adarsh Joshi h, Rebecca R. Hozak i, Ling Yang h, Jonathan D. Schwartz h,2, Celestia S. Higano j,1 a University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, United States b Memorial Sloan-Kettering, New York, NY, United States c University of Wisconsin, Carbone Cancer Center, Madison, WI, United States d Duke Cancer Institute and Duke Prostate Center, Duke University, Durham, NC, United States e Thomas Jefferson University, Philadelphia, PA, United States f New York University Clinical Cancer Center, New York, NY, United States g Sarah Cannon Research Institute, Nashville, TN, United States h Eli Lilly and Company, Bridgewater, NJ, United States i Eli Lilly and Company, Indianapolis, IN, United States j University of Washington, Fred Hutchinson Cancer Research Center, Seattle, WA, United States Received 11 February 2015; received in revised form 27 April 2015; accepted 10 May 2015 Available online 13 June 2015 KEYWORDS Abstract Background: Cixutumumab, a human monoclonal antibody (HuMAb), targets the Ramucirumab insulin-like growth factor receptor. Ramucirumab is a recombinant HuMAb that binds to vas- Cixutumumab cular endothelial growth factor receptor-2. A non-comparative randomised phase II study Mitoxantrone evaluated cixutumumab or ramucirumab plus mitoxantrone and prednisone (MP) in Prednisone metastatic castration-resistant prostate cancer (mCRPC). -

Research in Your Backyard Developing Cures, Creating Jobs
Research in Your Backyard Developing Cures, Creating Jobs PHARMACEUTICAL CLINICAL TRIALS IN ILLINOIS Dots show locations of clinical trials in the state. Executive Summary This report shows that biopharmaceutical research com- Quite often, biopharmaceutical companies hire local panies continue to be vitally important to the economy research institutions to conduct the tests and in Illinois, and patient health in Illinois, despite the recession. they help to bolster local economies in communities all over the state, including Chicago, Decatur, Joliet, Peoria, At a time when the state still faces significant economic Quincy, Rock Island, Rockford and Springfield. challenges, biopharmaceutical research companies are conducting or have conducted more than 4,300 clinical For patients, the trials offer another potential therapeutic trials of new medicines in collaboration with the state’s option. Clinical tests may provide a new avenue of care for clinical research centers, university medical schools and some chronic disease sufferers who are still searching for hospitals. Of the more than 4,300 clinical trials, 2,334 the medicines that are best for them. More than 470 of the target or have targeted the nation’s six most debilitating trials underway in Illinois are still recruiting patients. chronic diseases—asthma, cancer, diabetes, heart dis- ease, mental illnesses and stroke. Participants in clinical trials can: What are Clinical Trials? • Play an active role in their health care. • Gain access to new research treatments before they In the development of new medicines, clinical trials are are widely available. conducted to prove therapeutic safety and effectiveness and compile the evidence needed for the Food and Drug • Obtain expert medical care at leading health care Administration to approve treatments. -

(CHMP) Agenda for the Meeting on 22-25 February 2021 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes
22 February 2021 EMA/CHMP/107904/2021 Human Medicines Division Committee for medicinal products for human use (CHMP) Agenda for the meeting on 22-25 February 2021 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes 22 February 2021, 09:00 – 19:30, room 1C 23 February 2021, 08:30 – 19:30, room 1C 24 February 2021, 08:30 – 19:30, room 1C 25 February 2021, 08:30 – 19:30, room 1C Disclaimers Some of the information contained in this agenda is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the CHMP meeting highlights once the procedures are finalised and start of referrals will also be available. Of note, this agenda is a working document primarily designed for CHMP members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the agenda cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006). Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2021. -

Cytotoxic Chemotherapy: Still the Mainstay of Clinical Practice for All
G Model ONCH-2130; No. of Pages 14 ARTICLE IN PRESS Critical Reviews in Oncology/Hematology xxx (2016) xxx–xxx Contents lists available at ScienceDirect Critical Reviews in Oncology/Hematology journal homepage: www.elsevier.com/locate/critrevonc Cytotoxic chemotherapy: Still the mainstay of clinical practice for all subtypes metastatic breast cancer a,b b,∗ c c Chris Twelves , Maria Jove , Andrea Gombos , Ahmad Awada a Leeds Institute of Cancer and Pathology, St. James’s University Hospital, Leeds, UK b St James’s Institute of Oncology, St. James’s University Hospital, Bexley Wing, Level 4, Beckett Street, LS9 7TF Leeds, UK c Medical Oncology Clinic, Jules Bordet Institute, Université Libre de Bruxelles, Boulevard de Waterloo 121, B-1000 Brussels, Belgium Contents 1. Introduction . 00 2. Current therapeutic options for anthracycline- and taxane pretreated MBC . 00 2.1. Rechallenge with, or reformulation of, an anthracyclines or taxane . 00 2.2. Capecitabine . 00 3. Newer antimicrotubule agents . 00 3.1. Ixabepilone . 00 3.2. Eribulin . 00 4. Emerging new agents . 00 4.1. Vinflunine . 00 4.2. Etirinotecan pegol (NKTR-102). .00 4.2.1. Pharmacology. .00 4.2.2. Early clinical trials . 00 4.2.3. The BEACON trial . 00 5. Conclusions . 00 Conflict of interest . 00 Acknowledgments . 00 References . 00 Biography . 00 a r t i c l e i n f o a b s t r a c t Article history: Cytotoxic chemotherapy remains central to the treatment of all subtypes of metastatic breast cancer Received 13 October 2015 (MBC). We review evidence-based chemotherapy options for women with MBC after an anthracycline Received in revised form and a taxane including re-challenge with anthracycline or taxane, capecitabine, eribulin and ixabepilone 24 December 2015 as a single agent or combination with capecitabine (not approved in the EU); and the vinca alkaloid Accepted 20 January 2016 vinflunine as single agent or combined with either capecitabine/gemcitabine (also not approved EU or USA). -

Two Inhibitors of Yeast Plasma Membrane Atpase 1 (Scpma1p): Toward the Development of Novel Antifungal Therapies Sabine Ottilie1†, Gregory M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by D-Scholarship@Pitt Ottilie et al. J Cheminform (2018) 10:6 https://doi.org/10.1186/s13321-018-0261-3 RESEARCH ARTICLE Open Access Two inhibitors of yeast plasma membrane ATPase 1 (ScPma1p): toward the development of novel antifungal therapies Sabine Ottilie1†, Gregory M. Goldgof1,4†, Andrea L. Cheung1, Jennifer L. Walker2, Edgar Vigil1, Kenneth E. Allen3, Yevgeniya Antonova‑Koch1, Carolyn W. Slayman3^, Yo Suzuki4 and Jacob D. Durrant2* Abstract Given that many antifungal medications are susceptible to evolved resistance, there is a need for novel drugs with unique mechanisms of action. Inhibiting the essential proton pump Pma1p, a P-type ATPase, is a potentially efective therapeutic approach that is orthogonal to existing treatments. We identify NSC11668 and hitachimycin as structur‑ ally distinct antifungals that inhibit yeast ScPma1p. These compounds provide new opportunities for drug discovery aimed at this important target. Keywords: Antifungal, PMA1, P-type ATPase, Computer modeling, Saccharomyces cerevisiae, In vitro evolution, Drug resistance Background sterol-C-24-methyltransferase and the fungal cell mem- Antifungal medications are in high demand, but low brane directly [8]. efcacy, host toxicity, and emerging resistance among Only a few approved antimycotics have mecha- clinical strains [1, 2] complicate their use. Tere is an nisms that are unrelated to ergosterol biosynthesis. urgent need for novel antimycotic therapeutics with For example, the highly efective echinocandins inhibit unique mechanisms of action. Te purpose of the cur- 1,3-β-glucan synthase, hindering production of the criti- rent work is to describe two novel antifungals: 4-N,6- cal cell-wall component β-glucan [9, 10]; and the terato- N-bis(3-chlorophenyl)-1-methylpyrazolo[3,4-d] genic compound fucytosine interferes with eukaryotic pyrimidine-4,6-diamine (NSC11668), and hitachimycin RNA/DNA synthesis [11, 12]. -

Management of Brain and Leptomeningeal Metastases from Breast Cancer
International Journal of Molecular Sciences Review Management of Brain and Leptomeningeal Metastases from Breast Cancer Alessia Pellerino 1,* , Valeria Internò 2 , Francesca Mo 1, Federica Franchino 1, Riccardo Soffietti 1 and Roberta Rudà 1,3 1 Department of Neuro-Oncology, University and City of Health and Science Hospital, 10126 Turin, Italy; [email protected] (F.M.); [email protected] (F.F.); riccardo.soffi[email protected] (R.S.); [email protected] (R.R.) 2 Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro, 70121 Bari, Italy; [email protected] 3 Department of Neurology, Castelfranco Veneto and Treviso Hospital, 31100 Treviso, Italy * Correspondence: [email protected]; Tel.: +39-011-6334904 Received: 11 September 2020; Accepted: 10 November 2020; Published: 12 November 2020 Abstract: The management of breast cancer (BC) has rapidly evolved in the last 20 years. The improvement of systemic therapy allows a remarkable control of extracranial disease. However, brain (BM) and leptomeningeal metastases (LM) are frequent complications of advanced BC and represent a challenging issue for clinicians. Some prognostic scales designed for metastatic BC have been employed to select fit patients for adequate therapy and enrollment in clinical trials. Different systemic drugs, such as targeted therapies with either monoclonal antibodies or small tyrosine kinase molecules, or modified chemotherapeutic agents are under investigation. Major aims are to improve the penetration of active drugs through the blood–brain barrier (BBB) or brain–tumor barrier (BTB), and establish the best sequence and timing of radiotherapy and systemic therapy to avoid neurocognitive impairment. Moreover, pharmacologic prevention is a new concept driven by the efficacy of targeted agents on macrometastases from specific molecular subgroups. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known.