Niche Differentiation, Rarity, and Commonness in the Australian
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Musky Rat-Kangaroos, Hypsiprymnodon Moschatus: Cursorial Frugivores in Australia's Wet-Tropical Rain Forests
ResearchOnline@JCU This file is part of the following reference: Dennis, Andrew James (1997) Musky Rat-kangaroos, hypsiprymnodon moschatus: cursorial frugivores in Australia's wet-tropical rain forests. PhD thesis, James Cook University. Access to this file is available from: http://eprints.jcu.edu.au/17401/ If you believe that this work constitutes a copyright infringement, please contact [email protected] and quote http://eprints.jcu.edu.au/17401/ Chapter 11 MUSKY RAT-KANGAROOS: CURSORIAL FRUGIVORES How do Musky Rat-kangaroos Relate !Q..their Environment ? Musky Rat-kangaroos can be classified as frugivo res because fr ui ts and seeds accounted fo r the bul k of their diet th roughout the year (Chapter 3). In addi tion, they scatterhoarded many fruits and seeds, to the benefit of at least some species of plants (Chapters 8 & 9). They consumed most of the available frui ts which had a fleshy pericarp or ani but also included the seeds of some species that did not. They ate fruits from over half the species producing fruits on my study site, many of those they did not eat were wind dispersed, housed in hard, indehiscent pods or had furry. dehiscent pods. In addition, some fleshy drupes were not consumed. Like many other frugivores, Musky Rat-kangaroos supplemented their diet from other sources, particularly when the seasonal availabil ity of fruits is at its min imum (see Terborgh 1983). During the late wet, co ld an d early dry seasons, when frui t abundance was mini mal, their search effort was random with respect to fr ui t fall s (Figure 3.8; Table 3.2). -

A 'Slow Pace of Life' in Australian Old-Endemic Passerine Birds Is Not Accompanied by Low Basal Metabolic Rates
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers: part A Faculty of Science, Medicine and Health 1-1-2016 A 'slow pace of life' in Australian old-endemic passerine birds is not accompanied by low basal metabolic rates Claus Bech University of Wollongong Mark A. Chappell University of Wollongong, [email protected] Lee B. Astheimer University of Wollongong, [email protected] Gustavo A. Londoño Universidad Icesi William A. Buttemer University of Wollongong, [email protected] Follow this and additional works at: https://ro.uow.edu.au/smhpapers Part of the Medicine and Health Sciences Commons, and the Social and Behavioral Sciences Commons Recommended Citation Bech, Claus; Chappell, Mark A.; Astheimer, Lee B.; Londoño, Gustavo A.; and Buttemer, William A., "A 'slow pace of life' in Australian old-endemic passerine birds is not accompanied by low basal metabolic rates" (2016). Faculty of Science, Medicine and Health - Papers: part A. 3841. https://ro.uow.edu.au/smhpapers/3841 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] A 'slow pace of life' in Australian old-endemic passerine birds is not accompanied by low basal metabolic rates Abstract Life history theory suggests that species experiencing high extrinsic mortality rates allocate more resources toward reproduction relative to self-maintenance and reach maturity earlier ('fast pace of life') than those having greater life expectancy and reproducing at a lower rate ('slow pace of life'). Among birds, many studies have shown that tropical species have a slower pace of life than temperate-breeding species. -

Platypus Collins, L.R
AUSTRALIAN MAMMALS BIOLOGY AND CAPTIVE MANAGEMENT Stephen Jackson © CSIRO 2003 All rights reserved. Except under the conditions described in the Australian Copyright Act 1968 and subsequent amendments, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, duplicating or otherwise, without the prior permission of the copyright owner. Contact CSIRO PUBLISHING for all permission requests. National Library of Australia Cataloguing-in-Publication entry Jackson, Stephen M. Australian mammals: Biology and captive management Bibliography. ISBN 0 643 06635 7. 1. Mammals – Australia. 2. Captive mammals. I. Title. 599.0994 Available from CSIRO PUBLISHING 150 Oxford Street (PO Box 1139) Collingwood VIC 3066 Australia Telephone: +61 3 9662 7666 Local call: 1300 788 000 (Australia only) Fax: +61 3 9662 7555 Email: [email protected] Web site: www.publish.csiro.au Cover photos courtesy Stephen Jackson, Esther Beaton and Nick Alexander Set in Minion and Optima Cover and text design by James Kelly Typeset by Desktop Concepts Pty Ltd Printed in Australia by Ligare REFERENCES reserved. Chapter 1 – Platypus Collins, L.R. (1973) Monotremes and Marsupials: A Reference for Zoological Institutions. Smithsonian Institution Press, rights Austin, M.A. (1997) A Practical Guide to the Successful Washington. All Handrearing of Tasmanian Marsupials. Regal Publications, Collins, G.H., Whittington, R.J. & Canfield, P.J. (1986) Melbourne. Theileria ornithorhynchi Mackerras, 1959 in the platypus, 2003. Beaven, M. (1997) Hand rearing of a juvenile platypus. Ornithorhynchus anatinus (Shaw). Journal of Wildlife Proceedings of the ASZK/ARAZPA Conference. 16–20 March. -

Calaby References
Abbott, I.J. (1974). Natural history of Curtis Island, Bass Strait. 5. Birds, with some notes on mammal trapping. Papers and Proceedings of the Royal Society of Tasmania 107: 171–74. General; Rodents; Abbott, I. (1978). Seabird islands No. 56 Michaelmas Island, King George Sound, Western Australia. Corella 2: 26–27. (Records rabbit and Rattus fuscipes). General; Rodents; Lagomorphs; Abbott, I. (1981). Seabird Islands No. 106 Mondrain Island, Archipelago of the Recherche, Western Australia. Corella 5: 60–61. (Records bush-rat and rock-wallaby). General; Rodents; Abbott, I. and Watson, J.R. (1978). The soils, flora, vegetation and vertebrate fauna of Chatham Island, Western Australia. Journal of the Royal Society of Western Australia 60: 65–70. (Only mammal is Rattus fuscipes). General; Rodents; Adams, D.B. (1980). Motivational systems of agonistic behaviour in muroid rodents: a comparative review and neural model. Aggressive Behavior 6: 295–346. Rodents; Ahern, L.D., Brown, P.R., Robertson, P. and Seebeck, J.H. (1985). Application of a taxon priority system to some Victorian vertebrate fauna. Fisheries and Wildlife Service, Victoria, Arthur Rylah Institute of Environmental Research Technical Report No. 32: 1–48. General; Marsupials; Bats; Rodents; Whales; Land Carnivores; Aitken, P. (1968). Observations on Notomys fuscus (Wood Jones) (Muridae-Pseudomyinae) with notes on a new synonym. South Australian Naturalist 43: 37–45. Rodents; Aitken, P.F. (1969). The mammals of the Flinders Ranges. Pp. 255–356 in Corbett, D.W.P. (ed.) The natural history of the Flinders Ranges. Libraries Board of South Australia : Adelaide. (Gives descriptions and notes on the echidna, marsupials, murids, and bats recorded for the Flinders Ranges; also deals with the introduced mammals, including the dingo). -

Thylacinidae
FAUNA of AUSTRALIA 20. THYLACINIDAE JOAN M. DIXON 1 Thylacine–Thylacinus cynocephalus [F. Knight/ANPWS] 20. THYLACINIDAE DEFINITION AND GENERAL DESCRIPTION The single member of the family Thylacinidae, Thylacinus cynocephalus, known as the Thylacine, Tasmanian Tiger or Wolf, is a large carnivorous marsupial (Fig. 20.1). Generally sandy yellow in colour, it has 15 to 20 distinct transverse dark stripes across the back from shoulders to tail. While the large head is reminiscent of the dog and wolf, the tail is long and characteristically stiff and the legs are relatively short. Body hair is dense, short and soft, up to 15 mm in length. Body proportions are similar to those of the Tasmanian Devil, Sarcophilus harrisii, the Eastern Quoll, Dasyurus viverrinus and the Tiger Quoll, Dasyurus maculatus. The Thylacine is digitigrade. There are five digital pads on the forefoot and four on the hind foot. Figure 20.1 Thylacine, side view of the whole animal. (© ABRS)[D. Kirshner] The face is fox-like in young animals, wolf- or dog-like in adults. Hairs on the cheeks, above the eyes and base of the ears are whitish-brown. Facial vibrissae are relatively shorter, finer and fewer than in Tasmanian Devils and Quolls. The short ears are about 80 mm long, erect, rounded and covered with short fur. Sexual dimorphism occurs, adult males being larger on average. Jaws are long and powerful and the teeth number 46. In the vertebral column there are only two sacrals instead of the usual three and from 23 to 25 caudal vertebrae rather than 20 to 21. -
Mammals of Fleurieu Peninsula This List of Mammals of Fleurieu Peninsula Was Applied by the Late P.F
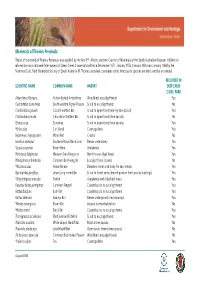
Mammals of Fleurieu Peninsula This list of mammals of Fleurieu Peninsula was applied by the late P.F. Aitken, onetime Curator of Mammals at the South Australian Museum. Additional information was obtained from surveys of Deep Creek Conservation Park in December 1971, January 1972, January 1980 and January 1984 by the Mammal Club, Field Naturalists Society of South Australia (R. Thomas, personal communication). Introduced species are indicated by an asterisk. RECORDED IN SCIENTIFIC NAME COMMON NAME HABITAT DEEP CREEK CONS. PARK Antechinus Flavipes Yellow-footed Antechinus Woodland, eucalypt forest Yes Cercartetus concinnus South western Pigmy Possum Scrub to eucalypt forest No Chalinolobus gouldii Gould's wattled Bat Scrub to open-forest (mainly tree spouts) Yes Chalinolobus moria Chocolate Wattled Bat Scrub to open-forest (tree spouts) No Eptesicus sp. Eptesicus Scrub to open-forest (tree spouts) Yes *Felis calus Cat (feral) Cosmopolitan Yes Hydromys chrysogaster Water Rat Creeks No Isoodon obesulus Southern Brown Bandicoot Dense understorey Yes *Lepus capensis Brown Hare Grasslands Yes Macropus fuliginosus Western Grey Kangaroo Heath to eucalypt forest Yes Miniopterus schreibersii Common Bent-wing Ba Eucalypt forest (caves) No *Mus musculus House Mouse Disturbed areas and early fire succession Yes Nyctophilus geoffroy Lesser Long-eared Bat Scrub to forest, semi cleared pasture (tree spouts, buildings) Yes *Oryctolagus cuniculus Rabbit Grasslands and disturbed areas Yes Pseudocheirus peregrinus Common Ringtail Coastal scrub to eucalypt -

Natural History of the Eutheria
FAUNA of AUSTRALIA 35. NATURAL HISTORY OF THE EUTHERIA P. J. JARMAN, A. K. LEE & L. S. HALL (with thanks for help to J.H. Calaby, G.M. McKay & M.M. Bryden) 1 35. NATURAL HISTORY OF THE EUTHERIA 2 35. NATURAL HISTORY OF THE EUTHERIA INTRODUCTION Unlike the Australian metatherian species which are all indigenous, terrestrial and non-flying, the eutherians now found in the continent are a mixture of indigenous and exotic species. Among the latter are some intentionally and some accidentally introduced species, and marine as well as terrestrial and flying as well as non-flying species are abundantly represented. All the habitats occupied by metatherians also are occupied by eutherians. Eutherians more than cover the metatherian weight range of 5 g–100 kg, but the largest terrestrial eutherians (which are introduced species) are an order of magnitude heavier than the largest extant metatherians. Before the arrival of dingoes 4000 years ago, however, none of the indigenous fully terrestrial eutherians weighed more than a kilogram, while most of the exotic species weigh more than that. The eutherians now represented in Australia are very diverse. They fall into major suites of species: Muridae; Chiroptera; marine mammals (whales, seals and dugong); introduced carnivores (Canidae and Felidae); introduced Leporidae (hares and rabbits); and introduced ungulates (Perissodactyla and Artiodactyla). In this chapter an attempt is made to compare and contrast the main features of the natural histories of these suites of species and, where appropriate, to comment on their resemblance to or difference from the metatherians. NATURAL HISTORY Ecology Diet. The native rodents are predominantly omnivorous. -

Regional Neutrality Evolves Through Local Adaptive Niche Evolution
Regional neutrality evolves through local adaptive niche evolution Mathew A. Leibolda,1, Mark C. Urbanb, Luc De Meesterc, Christopher A. Klausmeierd,e,f, and Joost Vanoverbekec aDepartment of Biology, University of Florida, Gainesville, FL 32611; bDepartment of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269; cDepartment of Biology, Katholieke Universiteit Leuven, B-3000 Leuven, Belgium; dKellogg Biological Station, Michigan State University, Hickory Corners, MI 49060; eDepartment of Plant Biology, Michigan State University, Hickory Corners, MI 49060; and fProgram in Ecology, Evolutionary Biology & Behavior, Michigan State University, Hickory Corners, MI 49060 Edited by Nils C. Stenseth, University of Oslo, Oslo, Norway, and approved December 11, 2018 (received for review May 22, 2018) Biodiversity in natural systems can be maintained either because Past theoretical work in this area suggests that, depending on niche differentiation among competitors facilitates stable coexis- assumptions, the effects of local adaptation can either cause com- tence or because equal fitness among neutral species allows for peting species to diverge (17) or converge (18–22) in niche traits, their long-term cooccurrence despite a slow drift toward extinc- facilitating niche partitioning or neutral cooccurrence of species, tion. Whereas the relative importance of these two ecological respectively. This research, however, neglects the regional scale mechanisms has been well-studied in the absence of evolution, the and the process by which communities assemble through re- role of local adaptive evolution in maintaining biological diversity peated colonization, extinction, and competition.Taking this through these processes is less clear. Here we study the contribu- more regional perspective, local adaptive evolution can generate tion of local adaptive evolution to coexistence in a landscape of evolution-mediated priority effects wherein early colonizers adapt to interconnected patches subject to disturbance. -

VAN DIEMEN's LAND COMPANY Records, 1824-1930 Reels M337
AUSTRALIAN JOINT COPYING PROJECT VAN DIEMEN’S LAND COMPANY Records, 1824-1930 Reels M337-64, M585-89 Van Diemen’s Land Company 35 Copthall Avenue London EC2 National Library of Australia State Library of New South Wales Filmed: 1960-61, 1964 CONTENTS Page 3 Historical note M337-64 4 Minutes of Court of Directors, 1824-1904 4 Outward letter books, 1825-1902 6 Inward letters and despatches, 1833-99 9 Letter books (Van Diemen’s Land), 1826-48 11 Miscellaneous papers, 1825-1915 14 Maps, plan and drawings, 1827-32 14 Annual reports, 1854-1922 M585-89 15 Legal documents, 1825-77 15 Accounts, 1833-55 16 Tasmanian letter books, 1848-59 17 Conveyances, 1851-1930 18 Miscellaneous papers 18 Index to despatches 2 HISTORICAL NOTE In 1824 a group of woollen mill owners, wool merchants, bankers and investors met in London to consider establishing a land company in Van Diemen’s Land similar to the Australian Agricultural Company in New South Wales. Encouraged by the support of William Sorell, the former Lieutenant- Governor, and Edward Curr, who had recently returned from the colony, they formed the Van Diemen’s Land Company and applied to Lord Bathurst for a grant of 500,000 acres. Bathurst agreed to a grant of 250,000 acres. The Van Diemen’s Land Company received a royal charter in 1825 giving it the right to cultivate land, build roads and bridges, lend money to colonists, execute public works, and build and buy houses, wharves and other buildings. Curr was appointed the chief agent of the Company in Van Diemen’s Land. -

Ba3444 MAMMAL BOOKLET FINAL.Indd
Intot Obliv i The disappearing native mammals of northern Australia Compiled by James Fitzsimons Sarah Legge Barry Traill John Woinarski Into Oblivion? The disappearing native mammals of northern Australia 1 SUMMARY Since European settlement, the deepest loss of Australian biodiversity has been the spate of extinctions of endemic mammals. Historically, these losses occurred mostly in inland and in temperate parts of the country, and largely between 1890 and 1950. A new wave of extinctions is now threatening Australian mammals, this time in northern Australia. Many mammal species are in sharp decline across the north, even in extensive natural areas managed primarily for conservation. The main evidence of this decline comes consistently from two contrasting sources: robust scientifi c monitoring programs and more broad-scale Indigenous knowledge. The main drivers of the mammal decline in northern Australia include inappropriate fi re regimes (too much fi re) and predation by feral cats. Cane Toads are also implicated, particularly to the recent catastrophic decline of the Northern Quoll. Furthermore, some impacts are due to vegetation changes associated with the pastoral industry. Disease could also be a factor, but to date there is little evidence for or against it. Based on current trends, many native mammals will become extinct in northern Australia in the next 10-20 years, and even the largest and most iconic national parks in northern Australia will lose native mammal species. This problem needs to be solved. The fi rst step towards a solution is to recognise the problem, and this publication seeks to alert the Australian community and decision makers to this urgent issue. -

Resource Competition Shapes Biological Rhythms and Promotes Temporal Niche
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.22.055160; this version posted April 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 2 3 Resource competition shapes biological rhythms and promotes temporal niche 4 differentiation in a community simulation 5 6 Resource competition, biological rhythms, and temporal niches 7 8 Vance Difan Gao1,2*, Sara Morley-Fletcher1,4, Stefania Maccari1,3,4, Martha Hotz Vitaterna2, Fred W. Turek2 9 10 1UMR 8576 Unité de Glycobiologie Structurale et Fonctionnelle, Campus Cité Scientifique, CNRS, University of 11 Lille, Lille, France 12 2 Center for Sleep and Circadian Biology, Northwestern University, Evanston, IL, United States of America 13 3Department of Medico-Surgical Sciences and Biotechnologies, University Sapienza of Rome, Rome, Italy 14 4International Associated Laboratory (LIA) “Perinatal Stress and Neurodegenerative Diseases”: University of Lille, 15 Lille, France; CNRS-UMR 8576, Lille, France; Sapienza University of Rome, Rome, Italy; IRCCS Neuromed, Pozzilli, 16 Italy 17 18 19 * Corresponding author 20 E-mail: [email protected] 21 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.04.22.055160; this version posted April 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. -

Interspecific Competition: • Lecture Summary: • Definition
BIOS 6150: Ecology Dr. Stephen Malcolm, Department of Biological Sciences • Week 5: Interspecific Competition: • Lecture summary: • Definition. Semibalanus balanoides • Examples. James P. Rowan, http://www.emature.com • Outcomes. • Lotka-Volterra model. Chthamalus stellatus Alan J. Southward, http://www.marlin.ac.uk/ BIOS 6150: Ecology - Dr. S. Malcolm. Week 5: Interspecific Competition Slide - 1 2. Interspecific Competition: • Like intraspecific competition, competition between species can be defined as: • “Competition is an interaction between individuals, brought about by a shared requirement for a resource in limited supply, and leading to a reduction in the survivorship, growth and/or reproduction of at least some of the competing individuals concerned” BIOS 6150: Ecology - Dr. S. Malcolm. Week 5: Interspecific Competition Slide - 2 3. Interspecific competition between 2 barnacle species (Fig. 8.2 after Connell, 1961): “Click for pictures” BIOS 6150: Ecology - Dr. S. Malcolm. Week 5: Interspecific Competition Slide - 3 4. Gause's Paramecium species compete interspecifically (Fig. 8.3): BIOS 6150: Ecology - Dr. S. Malcolm. Week 5: Interspecific Competition Slide - 4 5. Tilman's diatoms exploitation/scramble (Fig. 8.5): BIOS 6150: Ecology - Dr. S. Malcolm. Week 5: Interspecific Competition Slide - 5 6. A caveat: “The ghost of competition past:” • Lack observed 5 tit species in a single British wood: • 4 weighed 9.3-11.4g and 1 weighed 20.0g. • All have short beaks and hunt for insect food on leaves & twigs + seeds in winter. • Concluded that they coexisted because they exploited slightly different resources in slightly different ways. • But is this a justifiable explanation? Did species change or were species eliminated? • Connell (1980) emphasized that current patterns may be the product of past evolutionary responses to competition - “the ghost of competition past” ! BIOS 6150: Ecology - Dr.