Icd-10-Cm 2010 Tabular Addenda
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Clinical Management of Chronic Myelomonocytic Leukemia Eric Padron, MD, Rami Komrokji, and Alan F
The Clinical Management of Chronic Myelomonocytic Leukemia Eric Padron, MD, Rami Komrokji, and Alan F. List, MD Dr Padron is an assistant member, Dr Abstract: Chronic myelomonocytic leukemia (CMML) is an Komrokji is an associate member, and Dr aggressive malignancy characterized by peripheral monocytosis List is a senior member in the Department and ineffective hematopoiesis. It has been historically classified of Malignant Hematology at the H. Lee as a subtype of the myelodysplastic syndromes (MDSs) but was Moffitt Cancer Center & Research Institute in Tampa, Florida. recently demonstrated to be a distinct entity with a distinct natu- ral history. Nonetheless, clinical practice guidelines for CMML Address correspondence to: have been inferred from studies designed for MDSs. It is impera- Eric Padron, MD tive that clinicians understand which elements of MDS clinical Assistant Member practice are translatable to CMML, including which evidence has Malignant Hematology been generated from CMML-specific studies and which has not. H. Lee Moffitt Cancer Center & Research Institute This allows for an evidence-based approach to the treatment of 12902 Magnolia Drive CMML and identifies knowledge gaps in need of further study in Tampa, Florida 33612 a disease-specific manner. This review discusses the diagnosis, E-mail: [email protected] prognosis, and treatment of CMML, with the task of divorcing aspects of MDS practice that have not been demonstrated to be applicable to CMML and merging those that have been shown to be clinically similar. Introduction Chronic myelomonocytic leukemia (CMML) is a clonal hemato- logic malignancy characterized by absolute peripheral monocytosis, ineffective hematopoiesis, and an increased risk of transformation to acute myeloid leukemia. -

Poxviruses: Smallpox Vaccine, Its Complications and Chemotherapy
Virus Adaptation and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article R E V IEW Poxviruses: smallpox vaccine, its complications and chemotherapy Mimi Remichkova Abstract: The threat of bioterrorism in the recent years has once again posed to mankind the unresolved problems of contagious diseases, well forgotten in the past. Smallpox (variola) is Department of Pathogenic Bacteria, The Stephan Angeloff Institute among the most dangerous and highly contagious viral infections affecting humans. The last of Microbiology, Bulgarian Academy natural case in Somalia marked the end of a successful World Health Organization campaign of Sciences, Sofia, Bulgaria for smallpox eradication by vaccination on worldwide scale. Smallpox virus still exists today in some laboratories, specially designated for that purpose. The contemporary response in the treatment of the post-vaccine complications, which would occur upon enforcing new programs for mass-scale smallpox immunization, includes application of effective chemotherapeutics and their combinations. The goals are to provide the highest possible level of protection and safety of For personal use only. the population in case of eventual terrorist attack. This review describes the characteristic features of the poxviruses, smallpox vaccination, its adverse reactions, and poxvirus chemotherapy. Keywords: poxvirus, smallpox vaccine, post vaccine complications, inhibitors Characteristics of poxviruses Smallpox (variola) infection is caused by the smallpox virus. This virus belongs to the genus of Orthopoxvirus included in the Poxviridae family. Poxviruses are one of the largest and most complexly structured viruses, known so far. The genome of poxviruses consists of a linear two-chained DNA and its replication takes place in the cytoplasm of the infected cell. -
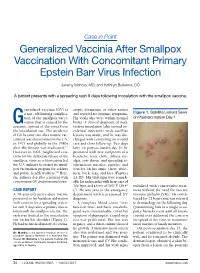
Generalized Vaccinia After Smallpox Vaccination with Concomitant Primary Epstein Barr Virus Infection
Case in Point Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection Jeremy Mandia, MD; and Kathryn Buikema, DO A patient presents with a spreading rash 9 days following inoculation with the smallpox vaccine. eneralized vaccinia (GV) is atopic dermatitis, or other rashes a rare, self-limiting complica- and reported no systemic symptoms. Figure 1. Satellite Lesions Seen tion of the smallpox vacci- His vitals also were within normal on Postvaccination Day 1 Gnation that is caused by the limits. A clinical diagnosis of inad- systemic spread of the virus from vertent inoculation (also termed ac- the inoculation site. The incidence cidental infection) with satellite of GV became rare after routine vac- lesions was made, and he was dis- cination was discontinued in the U.S. charged with counseling on wound in 1971 and globally in the 1980s care and close follow-up. Two days after the disease was eradicated.1,2 later, on postvaccination day 11, he However in 2002, heightened con- presented with new symptoms of a cerns for the deliberate release of the headache, fever, chills, diffuse my- smallpox virus as a bioweapon led algia, sore throat, and spreading er- the U.S. military to restart its small- ythematous macules, papules, and pox vaccination program for soldiers vesicles on his arms, chest, abdo- and public health workers.3,4 Here, men, back, legs, and face (Figures the authors describe a patient with 2A-2D). His vital signs were remark- concomitant GV and mononucleosis. able for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º stabilized with conservative treat- CASE REPORT C). -

Heater Element Specifications Bulletin Number 592
Technical Data Heater Element Specifications Bulletin Number 592 Topic Page Description 2 Heater Element Selection Procedure 2 Index to Heater Element Selection Tables 5 Heater Element Selection Tables 6 Additional Resources These documents contain additional information concerning related products from Rockwell Automation. Resource Description Industrial Automation Wiring and Grounding Guidelines, publication 1770-4.1 Provides general guidelines for installing a Rockwell Automation industrial system. Product Certifications website, http://www.ab.com Provides declarations of conformity, certificates, and other certification details. You can view or download publications at http://www.rockwellautomation.com/literature/. To order paper copies of technical documentation, contact your local Allen-Bradley distributor or Rockwell Automation sales representative. For Application on Bulletin 100/500/609/1200 Line Starters Heater Element Specifications Eutectic Alloy Overload Relay Heater Elements Type J — CLASS 10 Type P — CLASS 20 (Bul. 600 ONLY) Type W — CLASS 20 Type WL — CLASS 30 Note: Heater Element Type W/WL does not currently meet the material Type W Heater Elements restrictions related to EU ROHS Description The following is for motors rated for Continuous Duty: For motors with marked service factor of not less than 1.15, or Overload Relay Class Designation motors with a marked temperature rise not over +40 °C United States Industry Standards (NEMA ICS 2 Part 4) designate an (+104 °F), apply application rules 1 through 3. Apply application overload relay by a class number indicating the maximum time in rules 2 and 3 when the temperature difference does not exceed seconds at which it will trip when carrying a current equal to 600 +10 °C (+18 °F). -

Medical Management of Biological Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES FORT DETRICK FREDERICK, MARYLAND Emergency Response Numbers National Response Center: 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Helpline: 1-410-436-4484 or (Edgewood Ops Center – for military use) DSN 584-4484 USAMRIID’s Emergency Response Line: 1-888-872-7443 CDC'S Emergency Response Line: 1-770-488-7100 Handbook Download Site An Adobe Acrobat Reader (pdf file) version of this handbook can be downloaded from the internet at the following url: http://www.usamriid.army.mil USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 Lead Editor Lt Col Jon B. Woods, MC, USAF Contributing Editors CAPT Robert G. Darling, MC, USN LTC Zygmunt F. Dembek, MS, USAR Lt Col Bridget K. Carr, MSC, USAF COL Ted J. Cieslak, MC, USA LCDR James V. Lawler, MC, USN MAJ Anthony C. Littrell, MC, USA LTC Mark G. Kortepeter, MC, USA LTC Nelson W. Rebert, MS, USA LTC Scott A. Stanek, MC, USA COL James W. Martin, MC, USA Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 PREFACE TO THE SIXTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0221523 A1 Tseng Et Al
US 2009.022 1523A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0221523 A1 Tseng et al. (43) Pub. Date: Sep. 3, 2009 (54) NORTH-2'-DEOXY-METHANO- (86). PCT No.: PCT/US2O06/02O894 CARBATHYMIDNES AS ANTIVIRAL AGENTS AGAINST POXVIRUSES S371 (c)(1), (2), (4) Date: Apr. 3, 2009 (76) Inventors: Christopher K. Tseng, Related U.S. Application Data Burtonsville, MD (US); Victor E. (60) Provisional application No. 60/684.811, filed on May Marquez, Montgomery Village, 25, 2005. MD (US) Publication Classification (51) Int. Cl. Correspondence Address: A 6LX 3L/7072 (2006.01) KNOBBE, MARTENS, OLSON & BEAR, LLP A6IP3L/20 (2006.01) 2040 MAINSTREET, FOURTEENTH FLOOR (52) U.S. Cl. .......................................................... S14/SO IRVINE, CA 92.614 (US) (57) ABSTRACT (21) Appl. No.: 11/920,881 A method for the prevention or treatment of poxvirus infec tion by administering an effective amount of an antiviral agent comprising cyclopropanated carbocyclic 2'-deoxy (22) PCT Filed: May 25, 2006 nucleoside to an individual in need thereof is provided. Patent Application Publication Sep. 3, 2009 Sheet 2 of 3 US 2009/022 1523 A1 HSV-2 TK Goalpox TK humonl TK VV TK VOriod TK monkeypox TK fowlpox TK HSV-1 K african swine fever virus TK VZV TK EBV TK FIF2 Patent Application Publication Sep. 3, 2009 Sheet 3 of 3 US 2009/022 1523 A1 CC (N)-MCT CDV VC – -2 48.61 site - 2322 2027 . US 2009/022 1523 A1 Sep. 3, 2009 NORTH-2-DEOXY. breaks depended on the isolation of infected individuals and METHANOCARBATHYMIDNES AS the vaccination of close contacts. -

The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia
From www.bloodjournal.org by guest on January 9, 2019. For personal use only. Review Series THE UPDATED WHO CLASSIFICATION OF HEMATOLOGICAL MALIGNANCIES The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia Daniel A. Arber,1 Attilio Orazi,2 Robert Hasserjian,3 J¨urgen Thiele,4 Michael J. Borowitz,5 Michelle M. Le Beau,6 Clara D. Bloomfield,7 Mario Cazzola,8 and James W. Vardiman9 1Department of Pathology, Stanford University, Stanford, CA; 2Department of Pathology, Weill Cornell Medical College, New York, NY; 3Department of Pathology, Massachusetts General Hospital, Boston, MA; 4Institute of Pathology, University of Cologne, Cologne, Germany; 5Department of Pathology, Johns Hopkins Medical Institutions, Baltimore, MD; 6Section of Hematology/Oncology, University of Chicago, Chicago, IL; 7Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute, The Ohio State University, Columbus, OH; 8Department of Molecular Medicine, University of Pavia, and Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; and 9Department of Pathology, University of Chicago, Chicago, IL The World Health Organization (WHO) the prognostic relevance of entities cur- The 2016 edition represents a revision classification of tumors of the hemato- rently included in the WHO classification of the prior classification rather than poietic and lymphoid tissues was last and that also suggest new entities that an entirely new classification and at- updated in 2008. Since then, there have should be added. Therefore, there is a tempts to incorporate new clinical, prog- been numerous advances in the identifi- clear need for a revision to the current nostic, morphologic, immunophenotypic, cation of unique biomarkers associated classification. -

JOEL H. WESTRA, PH.D. ◊ Calvin University 3201 Burton St SE Grand Rapids, MI 49546 ◊ Tel: 616/526–6727 Email: [email protected] ◊ 24 May 2021
JOEL H. WESTRA, PH.D. ◊ Calvin University 3201 Burton St SE Grand Rapids, MI 49546 ◊ Tel: 616/526–6727 Email: [email protected] ◊ 24 May 2021 ACADEMIC APPOINTMENTS Associate Professor 2012–present Department of Politics and Economics – Calvin University Assistant Professor 2007–2012 Department of Politics and Economics – Calvin University Postdoctoral Fellow 2005–2007 John G. Tower Center for Political Studies – Southern Methodist University Visiting Lecturer 2004–2005 Committee on International Relations – The University of Chicago ADMINISTRATIVE APPOINTMENTS Department Chair 2016–present Department of Politics and Economics (co-chair) – Calvin University (2021–present) Department of Political Science – Calvin University (2016–2021) Program Director 2008–present Pre-Law Program – Calvin University Program Coordinator 2001–2003 Program on International Politics, Economics and Security (PIPES) – The University of Chicago EDUCATION Ph.D., Political Science 2004 Department of Political Science – The University of Chicago Subfields: International Relations, Political Theory, and Research Methods Dissertation Title: “Law, Power, and Argumentation: The UN Charter and Uses of Armed Force by Major Powers since 1945” Dissertation Committee: Duncan J. Snidal (chair), Robert A. Pape, Jack L. Goldsmith III M.A., Social Sciences 2000 Division of the Social Sciences – The University of Chicago Thesis Title: “Morality beyond the Polity: Political Realism and International Society from an Augustinian Perspective” Thesis Committee: Jean Bethke Elshtain (advisor), Lloyd Rudolph B.A., Political Science (Summa Cum Laude) 1998 Department of Political Science and the Honors College – University of Houston University Honors and Honors in Political Science Minors in Chemistry and Mathematics Thesis Title: “On the Moral Dilemma of Strategic Nuclear Weapons Targeting: An Exhumation of Jus in Bello Theory” Thesis Committee: Ross M. -

Structural and Functional Analysis of the Vaccinia Virus O1 Virulence Protein
Structural and functional analysis of the Vaccinia virus O1 virulence protein by Anastasia C. Weeks June 2017 Director of Thesis: Mark Mannie, Ph.D. Major Department: Biomedical Sciences, Microbiology and Immunology Poxviruses are double-stranded DNA viruses capable of causing disfiguring and deadly disease in a wide range of hosts, from insects to mammals. Orthopoxviruses (OPXV) encode many proteins that are not essential for viral replication, but are responsible for vast differences in pathogenesis. Of the >200 proteins in the prototypical OPXV vaccinia virus (VACV), many remain functionally cryptic. The objective of these studies was to understand how the VACV O1 protein functions by investigating cell-specific effects that may contribute to virulence. The O1L gene is expressed early as the O1 protein, a 78 kDa protein that lacked N-linked glycosylation. These data are the first to demonstrate the reduced ability of an O1 deletion mutant (∆O1) to induce cell migration compared to the parental VACV Western Reserve strain (VACV- WR). ∆O1-infected cell monolayers also exhibited reduced plaque diameter and clearance in plaque foci. These observations indicated that O1 is a significant contributor to VACV cytopathic effects (CPE) in vitro, in agreement with published reports. The results reported herein are the first to describe an altered immunological response with ∆O1, as levels of anti-VACV immunoglobulin significantly increased with ∆O1 infection at a time point (seven days post-infection) when VACV- WR induced VACV-specific antibody levels were comparable to sera from mock-infected mice. ∆O1 was more immunogenic in an ex vivo antigen presentation assay, although mitogen-induced CD4+ T cell activation during ∆O1 infection was equivalent to VACV-WR infection. -

Keyword Index
Neuropsychopharmacology (2014) 39, S692–S709 & 2014 American College of Neuropsychopharmacology. All rights reserved 0893-133X/14 www.neuropsychopharmacology.org Keyword Index 10q24.32 . ............... ................T156 Adolescent Depression . .M198 22q11....................................T37 adolescent development . M100, W223, M133 4-Chlorokynurenine . ....................... W226 adolescent stress . .........................M21 5-HT2A receptor . ....................... W196 ADRA1A . ........................T262 5-HT2C . ................................M130 adrenergic receptor . ....................... W205 5-HT3 receptors........................... W246 adult....................................W82 5-HT7 . ................................ M93 Adversity. ........................M199 5C-CPT . ................................W62 aerobic . ........................M163 5HT5A receptor antagonist....................W191 affective disorders . ........................W80 [11C]carfentanil............................M143 affective neuroscience . M167 affective priming . ........................M112 A African American . ........................T197 aggression . ........... M78, M211, T154, W74 aging..........W31, M181, M223, T54, W30, W31, W227, ABCB gene . ............................ W75 M141, M156, M232, W5, W104 ABCB1 . ................................T254 Agomelatine . ........................W210 Abuse Liability ........................... W160 Agonist . ....................... M266 Abuse Potential ............................W165 agoraphobia -

Oligomonocytic Chronic Myelomonocytic Leukemia
Modern Pathology (2017) 30, 1213–1222 © 2017 USCAP, Inc All rights reserved 0893-3952/17 $32.00 1213 Oligomonocytic chronic myelomonocytic leukemia (chronic myelomonocytic leukemia without absolute monocytosis) displays a similar clinicopathologic and mutational profile to classical chronic myelomonocytic leukemia Julia T Geyer1, Wayne Tam1, Yen-Chun Liu1, Zhengming Chen2, Sa A Wang3, Carlos Bueso-Ramos3, Jean Oak4, Daniel A Arber5, Eric Hsi6, Heesun J Rogers6, Katherine Levinson7, Adam Bagg7, Duane C Hassane1, Robert P Hasserjian8 and Attilio Orazi1 1Department of Pathology and Laboratory Medicine, Weill Cornell Medical College, New York, NY, USA; 2Division of Biostatistics and Epidemiology, Department of Healthcare Policy & Research, Weill Cornell Medical College, New York, NY, USA; 3Department of Hematopathology, the University of Texas MD Anderson Cancer Center, Houston, TX, USA; 4Department of Pathology, Stanford University, Stanford, CA, USA; 5Department of Pathology, University of Chicago, Chicago, IL, USA; 6Department of Laboratory Medicine, Cleveland Clinic, Cleveland, OH, USA; 7Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA, USA and 8Department of Pathology, Massachusetts General Hospital, Boston, MA, USA Chronic myelomonocytic leukemia is characterized by persistent absolute monocytosis (≥1×109/l) in the peripheral blood and dysplasia in ≥ 1 lineages. In the absence of dysplasia, an acquired clonal genetic abnormality is required or causes for reactive monocytosis have to be excluded.