The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Clinical Management of Chronic Myelomonocytic Leukemia Eric Padron, MD, Rami Komrokji, and Alan F
The Clinical Management of Chronic Myelomonocytic Leukemia Eric Padron, MD, Rami Komrokji, and Alan F. List, MD Dr Padron is an assistant member, Dr Abstract: Chronic myelomonocytic leukemia (CMML) is an Komrokji is an associate member, and Dr aggressive malignancy characterized by peripheral monocytosis List is a senior member in the Department and ineffective hematopoiesis. It has been historically classified of Malignant Hematology at the H. Lee as a subtype of the myelodysplastic syndromes (MDSs) but was Moffitt Cancer Center & Research Institute in Tampa, Florida. recently demonstrated to be a distinct entity with a distinct natu- ral history. Nonetheless, clinical practice guidelines for CMML Address correspondence to: have been inferred from studies designed for MDSs. It is impera- Eric Padron, MD tive that clinicians understand which elements of MDS clinical Assistant Member practice are translatable to CMML, including which evidence has Malignant Hematology been generated from CMML-specific studies and which has not. H. Lee Moffitt Cancer Center & Research Institute This allows for an evidence-based approach to the treatment of 12902 Magnolia Drive CMML and identifies knowledge gaps in need of further study in Tampa, Florida 33612 a disease-specific manner. This review discusses the diagnosis, E-mail: [email protected] prognosis, and treatment of CMML, with the task of divorcing aspects of MDS practice that have not been demonstrated to be applicable to CMML and merging those that have been shown to be clinically similar. Introduction Chronic myelomonocytic leukemia (CMML) is a clonal hemato- logic malignancy characterized by absolute peripheral monocytosis, ineffective hematopoiesis, and an increased risk of transformation to acute myeloid leukemia. -

The Association of Bladder Myeloid Sarcoma and Unclassified
90 Case Report The association of bladder myeloid sarcoma and unclassified myelodysplastic/myeloproliferative disease Mesanede myeloid sarkom ve sınıflandırılamayan myeloproliferatif/myelodisplastik hastalık birlikteliği Mehmet Sönmez1, Ümit Çobanoğlu2, Sevdagül Mungan2, Bircan Sönmez3, Rasin Özyavuz4 1Department of Hematology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 2Department of Pathology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 3Department of Nuclear Medicine, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 4Department of Urology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey Abstract Myeloid sarcoma of the urinary bladder is a rare disorder. We report a 71-year-old man with hematuria who had a diffuse myeloid sarcoma of the bladder. He was also under follow-up for unclassified myeloproliferative/myelodysplastic disorder, diagnosed two months before. Abdominal ultrasonography and computed tomography findings were normal. Diagnostic cystoscopy revealed patchy areas of mucosal swelling with hyperemia. Histopathological examination of biopsies demon- strated a neoplasm composed of blasts showing myeloperoxidase positivity by immunohistochemistry. To our knowledge, the current case is the first case of myeloid sarcoma in the urinary bladder without evidence of a mass lesion, with a concurrent diagnosis of unclassifiable myelodysplastic/myeloproliferative disease. (Turk J Hematol 2009; 26: 90-2) Key words: Myeloid sarcoma, urinary bladder, unclassified myelodysplastic/myeloproliferative disease Received: April 3, 2008 Accepted: September 10, 2008 Özet Myeloid sarkom mesanede nadir görülen bir hastalıktır. Bu vaka takdiminde hematüri ile başvuran ve 2 ay önce sınıflandırılamayan myeloproliferatif/myelodisplastik hastalık tanısı almış 71 yaşında erkek hastada mesanede diffüz tutulum ile seyreden myeloid sarkom tanımlandı. Hastanın batın ultrasonografisi ve tomografisi normal olup, tanısal amaçlı sistosko- pide hiperemik ve ödemli bir mukoza izlendi. -

The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Inherited Polyposis Syndromes Daniel Herzig, M.D
CLINICAL PRACTICE GUIDELINES The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Inherited Polyposis Syndromes Daniel Herzig, M.D. • Karin Hardimann, M.D. • Martin Weiser, M.D. • Nancy Yu, M.D. Ian Paquette, M.D. • Daniel L. Feingold, M.D. • Scott R. Steele, M.D. Prepared by the Clinical Practice Guidelines Committee of The American Society of Colon and Rectal Surgeons he American Society of Colon and Rectal Surgeons METHODOLOGY (ASCRS) is dedicated to ensuring high-quality pa- tient care by advancing the science, prevention, and These guidelines are built on the last set of the ASCRS T Practice Parameters for the Identification and Testing of management of disorders and diseases of the colon, rectum, Patients at Risk for Dominantly Inherited Colorectal Can- and anus. The Clinical Practice Guidelines Committee is 1 composed of society members who are chosen because they cer published in 2003. An organized search of MEDLINE have demonstrated expertise in the specialty of colon and (1946 to December week 1, 2016) was performed from rectal surgery. This committee was created to lead interna- 1946 through week 4 of September 2016 (Fig. 1). Subject tional efforts in defining quality care for conditions related headings for “adenomatous polyposis coli” (4203 results) to the colon, rectum, and anus, in addition to the devel- and “intestinal polyposis” (445 results) were included, us- opment of Clinical Practice Guidelines based on the best ing focused search. The results were combined (4629 re- available evidence. These guidelines are inclusive and not sults) and limited to English language (3981 results), then prescriptive. -

Hepatoblastoma and APC Gene Mutation in Familial Adenomatous Polyposis Gut: First Published As 10.1136/Gut.39.6.867 on 1 December 1996
Gut 1996; 39: 867-869 867 Hepatoblastoma and APC gene mutation in familial adenomatous polyposis Gut: first published as 10.1136/gut.39.6.867 on 1 December 1996. Downloaded from F M Giardiello, G M Petersen, J D Brensinger, M C Luce, M C Cayouette, J Bacon, S V Booker, S R Hamilton Abstract tumours; and extracolonic cancers of the Background-Hepatoblastoma is a rare, thyroid, duodenum, pancreas, liver, and rapidly progressive, usually fatal child- brain.1-4 hood malignancy, which if confined to the Hepatoblastoma is a rare malignant liver can be cured by radical surgical embryonal tumour of the liver, which occurs in resection. An association between hepato- infancy and childhood. An association between blastoma and familial adenomatous hepatoblastoma and familial adenomatous polyposis (FAP), which is due to germline polyposis was first described by Kingston et al mutation of the APC (adenomatous in 1982,6 and since then over 30 additional polyposis coli) gene, has been confirmed, cases have been reported.7-15 Moreover, a but correlation with site of APC mutation pronounced increased relative risk of hepato- has not been studied. blastoma in patients affected with FAP and Aim-To analyse the APC mutational their first degree relatives has been found spectrum in FAP families with hepato- (relative risk 847, 95% confidence limits 230 blastoma as a possible basis to select and 2168).16 kindreds for surveillance. FAP is caused by germline mutations of the Patients-Eight patients with hepato- APC (adenomatous polyposis coli) gene blastoma in seven FAP kindreds were located on the long arm of chromosome 5 in compared with 97 families with identified band q2 1.17-'20The APC gene has 15 exons and APC gene mutation in a large Registry. -

Treatment Outcomes of Pediatric Acute Myeloid Leukemia In
children Article Treatment Outcomes of Pediatric Acute Myeloid Leukemia in the Yeungnam Region: A Multicenter Retrospective Study of the Study Alliance of Yeungnam Pediatric Hematology–Oncology (SAYPH) Jae Min Lee 1 , Eu Jeen Yang 2 , Kyung Mi Park 2 , Young-Ho Lee 3, Heewon Chueh 4, Jeong Ok Hah 5, Ji Kyoung Park 6, Jae Young Lim 7, Eun Sil Park 7, Sang Kyu Park 8, Heung Sik Kim 9, Ye Jee Shim 10 , Jeong A. Park 11,12, Eun Jin Choi 13, Kun Soo Lee 14, Ji Yoon Kim 14 and Young Tak Lim 2,* 1 Department of Pediatrics, College of Medicine, Yeungnam University, Daegu 42415, Korea; [email protected] 2 Department of Pediatrics, Pusan National University Children’s Hospital, Pusan National University, School of Medicine, Yangsan 50612, Korea; [email protected] (E.J.Y.); [email protected] (K.M.P.) 3 Department of Pediatrics, Hanyang University College of Medicine, Hanyang University Medical Center, Seoul 04763, Korea; [email protected] 4 Department of Pediatrics, Dong-A University College of Medicine, Busan 49201, Korea; [email protected] 5 Department of Pediatrics, Daegu Fatima Hospital, Daegu 41199, Korea; [email protected] 6 Department of Pediatrics, Inje University College of Medicine, Busan Paik Hospital, Busan 47392, Korea; [email protected] 7 Department of Pediatrics, Gyeongsang National University College of Medicine, Jinju 52727, Korea; [email protected] (J.Y.L.); [email protected] (E.S.P.) Citation: Lee, J.M.; Yang, E.J.; Park, 8 Department of Pediatrics, Ulsan University Hospital, Ulsan 44033, Korea; [email protected] K.M.; Lee, Y.-H.; Chueh, H.; Hah, J.O.; 9 Department of Pediatrics, Keimyung University School of Medicine, Keimyung University Daegu Dongsan Park, J.K.; Lim, J.Y.; Park, E.S.; Park, Hospital, Daegu 41931, Korea; [email protected] S.K.; et al. -

Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D
American Journal of Gastroenterology ISSN 0002-9270 C 2006 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2006.00375.x Published by Blackwell Publishing CME Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D. Foulkes, M.B., Ph.D.2 1Division of Gastroenterology, Department of Medicine, The Sir Mortimer B. Davis Jewish General Hospital, McGill University, Montreal, Quebec, Canada, and 2Program in Cancer Genetics, Departments of Oncology and Human Genetics, McGill University, Montreal, Quebec, Canada Familial adenomatous polyposis (FAP) is an autosomal-dominant colorectal cancer syndrome, caused by a germline mutation in the adenomatous polyposis coli (APC) gene, on chromosome 5q21. It is characterized by hundreds of adenomatous colorectal polyps, with an almost inevitable progression to colorectal cancer at an average age of 35 to 40 yr. Associated features include upper gastrointestinal tract polyps, congenital hypertrophy of the retinal pigment epithelium, desmoid tumors, and other extracolonic malignancies. Gardner syndrome is more of a historical subdivision of FAP, characterized by osteomas, dental anomalies, epidermal cysts, and soft tissue tumors. Other specified variants include Turcot syndrome (associated with central nervous system malignancies) and hereditary desmoid disease. Several genotype–phenotype correlations have been observed. Attenuated FAP is a phenotypically distinct entity, presenting with fewer than 100 adenomas. Multiple colorectal adenomas can also be caused by mutations in the human MutY homologue (MYH) gene, in an autosomal recessive condition referred to as MYH associated polyposis (MAP). Endoscopic screening of FAP probands and relatives is advocated as early as the ages of 10–12 yr, with the objective of reducing the occurrence of colorectal cancer. -
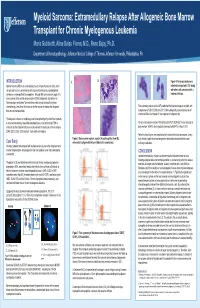
Myeloid Sarcoma
Myeloid Sarcoma: Extramedullary Relapse After Allogeneic Bone Marrow Transplant for Chronic Myelogenous Leukemia Maria Gubbiotti, Alina Dulau Florea, M.D., Renu Bajaj, Ph.D. Department of Hematopathology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA INTRODUCTION A. B. Figure 4: Numerous blasts were Myeloid sarcoma (MS) is an extramedullary tumor of myeloid precursor cells, which detected in the patient’s CSF along can precede or occur concomitantly with acute myeloid leukemia, myelodysplastic with other cells consistent with a syndrome, or myeloproliferative neoplasms. Although MS can involve any organ, it is leukemic infiltrate. more common in the central nervous system (CNS) and gonads, sites known as “pharmacologic sanctuaries” where leukemic cells can survive despite systemic chemotherapy. Less often, this tumor can be the manner of relapse after allogeneic Flow cytometry analysis of the CSF established the blast phenotype as myeloid, with bone marrow transplantation. coexpression of CD13, CD33 and CD117. She subsequently received two cycles of intrathecal ARA-C and repeat LP was negative for malignant cells. The diagnosis is based on morphology and immunophenotype by either flow cytometry or immunohistochemistry of paraffin-embedded tissue, and confirmed by FISH or Reverse transcription real-time PCR detected the P210 BCR-ABL1 fusion transcript in molecular studies. Myeloid sarcomas usually express the leukocyte common antigens bone marrow : 0.064%, which gradually increased to 98.947% in March 2013. CD45, CD13, CD33, CD43 and lack T-cell and B-cell antigens. Patient’s clinical course was complicated with multiple infections (pneumonia, urinary Figure 2: Bone marrow aspirate, regular (A) and magnified view (B), tract infection) septic shock and progressive deterioration despite antibiotics and Case Study demonstrating hypercellularity and blast crisis respectively. -

Cancer Treatment and Survivorship Facts & Figures 2019-2021
Cancer Treatment & Survivorship Facts & Figures 2019-2021 Estimated Numbers of Cancer Survivors by State as of January 1, 2019 WA 386,540 NH MT VT 84,080 ME ND 95,540 59,970 38,430 34,360 OR MN 213,620 300,980 MA ID 434,230 77,860 SD WI NY 42,810 313,370 1,105,550 WY MI 33,310 RI 570,760 67,900 IA PA NE CT 243,410 NV 185,720 771,120 108,500 OH 132,950 NJ 543,190 UT IL IN 581,350 115,840 651,810 296,940 DE 55,460 CA CO WV 225,470 1,888,480 KS 117,070 VA MO MD 275,420 151,950 408,060 300,200 KY 254,780 DC 18,750 NC TN 470,120 AZ OK 326,530 NM 207,260 AR 392,530 111,620 SC 143,320 280,890 GA AL MS 446,900 135,260 244,320 TX 1,140,170 LA 232,100 AK 36,550 FL 1,482,090 US 16,920,370 HI 84,960 States estimates do not sum to US total due to rounding. Source: Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute. Contents Introduction 1 Long-term Survivorship 24 Who Are Cancer Survivors? 1 Quality of Life 24 How Many People Have a History of Cancer? 2 Financial Hardship among Cancer Survivors 26 Cancer Treatment and Common Side Effects 4 Regaining and Improving Health through Healthy Behaviors 26 Cancer Survival and Access to Care 5 Concerns of Caregivers and Families 28 Selected Cancers 6 The Future of Cancer Survivorship in Breast (Female) 6 the United States 28 Cancers in Children and Adolescents 9 The American Cancer Society 30 Colon and Rectum 10 How the American Cancer Society Saves Lives 30 Leukemia and Lymphoma 12 Research 34 Lung and Bronchus 15 Advocacy 34 Melanoma of the Skin 16 Prostate 16 Sources of Statistics 36 Testis 17 References 37 Thyroid 19 Acknowledgments 45 Urinary Bladder 19 Uterine Corpus 21 Navigating the Cancer Experience: Treatment and Supportive Care 22 Making Decisions about Cancer Care 22 Cancer Rehabilitation 22 Psychosocial Care 23 Palliative Care 23 Transitioning to Long-term Survivorship 23 This publication attempts to summarize current scientific information about Global Headquarters: American Cancer Society Inc. -

Sporadic (Nonhereditary) Colorectal Cancer: Introduction
Sporadic (Nonhereditary) Colorectal Cancer: Introduction Colorectal cancer affects about 5% of the population, with up to 150,000 new cases per year in the United States alone. Cancer of the large intestine accounts for 21% of all cancers in the US, ranking second only to lung cancer in mortality in both males and females. It is, however, one of the most potentially curable of gastrointestinal cancers. Colorectal cancer is detected through screening procedures or when the patient presents with symptoms. Screening is vital to prevention and should be a part of routine care for adults over the age of 50 who are at average risk. High-risk individuals (those with previous colon cancer , family history of colon cancer , inflammatory bowel disease, or history of colorectal polyps) require careful follow-up. There is great variability in the worldwide incidence and mortality rates. Industrialized nations appear to have the greatest risk while most developing nations have lower rates. Unfortunately, this incidence is on the increase. North America, Western Europe, Australia and New Zealand have high rates for colorectal neoplasms (Figure 2). Figure 1. Location of the colon in the body. Figure 2. Geographic distribution of sporadic colon cancer . Symptoms Colorectal cancer does not usually produce symptoms early in the disease process. Symptoms are dependent upon the site of the primary tumor. Cancers of the proximal colon tend to grow larger than those of the left colon and rectum before they produce symptoms. Abnormal vasculature and trauma from the fecal stream may result in bleeding as the tumor expands in the intestinal lumen. -

VARYING DEGREES of MALIGNANCY in CANCER of the BREAST Differences in the Degree of Malignancy of Malignant Tumors Have Been Reco
VARYING DEGREES OF MALIGNANCY IN CANCER OF THE BREAST ROBERT 13. GREENOUGH BOSTON Differences in the degree of malignancy of malignant tumors have been recognized by pathologists for many years. Indeed, Virchow’s original conception of a malignant tumor was one composed of cells, derived from the tissue cells of the individual, but differing from the normal cells in the rapidity and independ- ence of their growth. Hansemann (1) carried this idea somewhat further and intro- duced the word “anaplasia” to indicate the process by which cancer cells came to differ from the normal type cell of the body tissue concerned. Anaplasia involves a loss of differentiation and an increase of reproductive power, so that the anaplastic cell fulfils only in abortive fashion, if at all, its normal function, such as secretion or keratinization; while it shows by increased number of mitotic figures, and especially by the irregularity and abnormality of its nuclear chromatic elements and figures, the increase in rapidity of cell division and of cell growth which is characteristic of malignancy. X number of attempts have been made to grade the malig- nancy of different breast tumors by distinguishing their histo- logical characteristics, such as adenocarcinoma, medullary, scirrhus, colloid, etc. ; but with the exception of adenocarcinoma and colloid, these divisions have proved of little value in prognosis. There the matter rested till 1921, when, under the influence of MacCarty (2) of the Mayo Clinic, Broders published a paper suggesting the classification of cancer tissue according to the degree of malignancy, as estimated by loss of differentiation and increase of reproductive characteristics. -

Early Pancreatic Cancers: Pearls, Pitfalls and Mimics
Early Pancreatic Cancers: Pearls, Pitfalls and Mimics H A Siddiki, MD, J G Fletcher, MD, N Takahashi, MD, J L Fidler, MD, N Dajani, MD, J E Huprich, MD, D M Hough, MD Department of Radiology, Mayo Clinic, Rochester, MN PURPOSE Overview and Test Cases Discussion Atypical Findings of Pancreatic Cancer Pitfalls in Tumor Detection Pancreatic Cancer Mimics •Autoimmune pancreatitis To display a spectrum of early • Isoattenuating mass • Sub-optimal scanning • Chronic pancreatitis and atypical presentations of • Exophytic tumors • Pancreatitis (acute or chronic) Atypical Findings Mimics • Perineural and perivascular infiltration • Metastases Isoattenuating Mass . Despite multiphasic, thin section CT, adenocarcinoma of the • Occult neoplasms Autoimmune Pancreatitis (AIP). A1 A2 approximately 10-15% of pancreatic adenocarcinomas are A3 Characteristic imaging findings of AIP • Neoplasms that mimic pancreatic cancer isoattenuating. In such instances secondary signs such as pancreatic without mass • Presence of a stent include diffuse pancreatic enlargement pancreas, in addition to imaging ductal dilation and cutoff, loss of fatty marbling, contour abnormality, or and/or capsule-like rim. Focal mass-like • Intrapancreatic splenule atrophic distal pancreatic parenchyma must be relied upon to visualize • Diffusely infiltrating tumors enlargement of the pancreas is not the mass. Similarly, some pancreatic cancers may demonstrate pitfalls and mimics, in a case- uncommon and may be indistinguishable isointense signal at MR. Case on the right with a 2 cm pancreatic head • Cystic change • Focal fat from pancreatic cancer. Extrapancreatic carcinoma. CT portovenous phase (right images) and MR post contrast based presentation and review. involvement of bile ducts (thickening or LAVA ( left images) show an isointense and isoattenuating mass. -

Compressive Myelopathy Caused by Isolated Epidural Myeloid Sarcoma with Systemic Mastocytosis
Clinical Notes Compressive myelopathy caused by isolated epidural myeloid sarcoma with systemic mastocytosis. Rare presentation of a hematological malignancy Cyril J. Kurian, MBBS, Indira Madhavan, MBBS, MD, Prabhalakshmi K. Krishnankutty, MBBS, MD, Mekkattukunnel A. Andrews, MD, DM. eurological manifestations of acute leukemia are Ndue to direct involvement by meningeal infiltration and myeloid sarcoma; and indirect involvement by immunosuppression and treatment related side effects. It is rare for myeloid sarcoma to present without bone marrow involvement (isolated myeloid sarcoma or primary granulocytic sarcoma).1 It is even rarer for an isolated myeloid sarcoma to present in the epidural space. We evaluated a case of paraplegia admitted to our department. He had several atypical features that we would like to present in this report. A 39-year-old gentleman with a body weight of Figure 1 - Magnetic resonance imaging of thoracic spine T1W sagittal 58 kg presented with paresthesia and heaviness of view, arrow showing extradural mass at T6 level. both lower limbs of 4 days duration. He was found to have spastic paraplegia with bladder involvement and sensory level at T6. The clinical diagnosis of acute peripheral blood picture showed dimorphic anemia, transverse myelitis was made. Table 1 summarizes the occasional large cells with granular cytoplasm and nucleus with condensed chromatin, and no blast cells. laboratory investigations. The MRI study of the dorsal Ultrasound of the abdomen showed mild splenomegaly. spine (Figure 1) shows that a moderate sized enhancing Urine Bence Jones protein was absent. No M band was posterior epidural component was compressing the seen on serum protein electrophoresis. Bone marrow thecal sac and spinal cord.