Mast Cell Sarcoma: a Rare and Potentially Under
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Updates in Mastocytosis
Updates in Mastocytosis Tryptase PD-L1 Tracy I. George, M.D. Professor of Pathology 1 Disclosure: Tracy George, M.D. Research Support / Grants None Stock/Equity (any amount) None Consulting Blueprint Medicines Novartis Employment ARUP Laboratories Speakers Bureau / Honoraria None Other None Outline • Classification • Advanced mastocytosis • A case report • Clinical trials • Other potential therapies Outline • Classification • Advanced mastocytosis • A case report • Clinical trials • Other potential therapies Mastocytosis symposium and consensus meeting on classification and diagnostic criteria for mastocytosis Boston, October 25-28, 2012 2008 WHO Classification Scheme for Myeloid Neoplasms Acute Myeloid Leukemia Chronic Myelomonocytic Leukemia Atypical Chronic Myeloid Leukemia Juvenile Myelomonocytic Leukemia Myelodysplastic Syndromes MDS/MPN, unclassifiable Chronic Myelogenous Leukemia MDS/MPN Polycythemia Vera Essential Thrombocythemia Primary Myelofibrosis Myeloproliferative Neoplasms Chronic Neutrophilic Leukemia Chronic Eosinophilic Leukemia, NOS Hypereosinophilic Syndrome Mast Cell Disease MPNs, unclassifiable Myeloid or lymphoid neoplasms Myeloid neoplasms associated with PDGFRA rearrangement associated with eosinophilia and Myeloid neoplasms associated with PDGFRB abnormalities of PDGFRA, rearrangement PDGFRB, or FGFR1 Myeloid neoplasms associated with FGFR1 rearrangement (EMS) 2017 WHO Classification Scheme for Myeloid Neoplasms Chronic Myelomonocytic Leukemia Acute Myeloid Leukemia Atypical Chronic Myeloid Leukemia Juvenile Myelomonocytic -

The Association of Bladder Myeloid Sarcoma and Unclassified
90 Case Report The association of bladder myeloid sarcoma and unclassified myelodysplastic/myeloproliferative disease Mesanede myeloid sarkom ve sınıflandırılamayan myeloproliferatif/myelodisplastik hastalık birlikteliği Mehmet Sönmez1, Ümit Çobanoğlu2, Sevdagül Mungan2, Bircan Sönmez3, Rasin Özyavuz4 1Department of Hematology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 2Department of Pathology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 3Department of Nuclear Medicine, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 4Department of Urology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey Abstract Myeloid sarcoma of the urinary bladder is a rare disorder. We report a 71-year-old man with hematuria who had a diffuse myeloid sarcoma of the bladder. He was also under follow-up for unclassified myeloproliferative/myelodysplastic disorder, diagnosed two months before. Abdominal ultrasonography and computed tomography findings were normal. Diagnostic cystoscopy revealed patchy areas of mucosal swelling with hyperemia. Histopathological examination of biopsies demon- strated a neoplasm composed of blasts showing myeloperoxidase positivity by immunohistochemistry. To our knowledge, the current case is the first case of myeloid sarcoma in the urinary bladder without evidence of a mass lesion, with a concurrent diagnosis of unclassifiable myelodysplastic/myeloproliferative disease. (Turk J Hematol 2009; 26: 90-2) Key words: Myeloid sarcoma, urinary bladder, unclassified myelodysplastic/myeloproliferative disease Received: April 3, 2008 Accepted: September 10, 2008 Özet Myeloid sarkom mesanede nadir görülen bir hastalıktır. Bu vaka takdiminde hematüri ile başvuran ve 2 ay önce sınıflandırılamayan myeloproliferatif/myelodisplastik hastalık tanısı almış 71 yaşında erkek hastada mesanede diffüz tutulum ile seyreden myeloid sarkom tanımlandı. Hastanın batın ultrasonografisi ve tomografisi normal olup, tanısal amaçlı sistosko- pide hiperemik ve ödemli bir mukoza izlendi. -

Diagnostic Immunohistochemistry for Canine Cutaneous Round Cell Tumours — Retrospective Analysis of 60 Cases
FOLIA HISTOCHEMICA ORIGINAL PAPER ET CYTOBIOLOGICA Vol. 57, No. 3, 2019 pp. 146–154 Diagnostic immunohistochemistry for canine cutaneous round cell tumours — retrospective analysis of 60 cases Katarzyna Pazdzior-Czapula, Mateusz Mikiewicz, Michal Gesek, Cezary Zwolinski, Iwona Otrocka-Domagala Department of Pathological Anatomy, Faculty of Veterinary Medicine, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland Abstract Introduction. Canine cutaneous round cell tumours (CCRCTs) include various benign and malignant neoplastic processes. Due to their similar morphology, the diagnosis of CCRCTs based on histopathological examination alone can be challenging, often necessitating ancillary immunohistochemical (IHC) analysis. This study presents a retrospective analysis of CCRCTs. Materials and methods. This study includes 60 cases of CCRCTs, including 55 solitary and 5 multiple tumours, evaluated immunohistochemically using a basic antibody panel (MHCII, CD18, Iba1, CD3, CD79a, CD20 and mast cell tryptase) and, when appropriate, extended antibody panel (vimentin, desmin, a-SMA, S-100, melan-A and pan-keratin). Additionally, histochemical stainings (May-Grünwald-Giemsa and methyl green pyronine) were performed. Results. IHC analysis using a basic antibody panel revealed 27 cases of histiocytoma, one case of histiocytic sarcoma, 18 cases of cutaneous lymphoma of either T-cell (CD3+) or B-cell (CD79a+) origin, 5 cases of plas- macytoma, and 4 cases of mast cell tumours. The extended antibody panel revealed 2 cases of alveolar rhabdo- myosarcoma, 2 cases of amelanotic melanoma, and one case of glomus tumour. Conclusions. Both canine cutaneous histiocytoma and cutaneous lymphoma should be considered at the beginning of differential diagnosis for CCRCTs. While most poorly differentiated CCRCTs can be diagnosed immunohis- tochemically using 1–4 basic antibodies, some require a broad antibody panel, including mesenchymal, epithelial, myogenic, and melanocytic markers. -

PROPOSED REGULATION of the STATE BOARD of HEALTH LCB File No. R057-16
PROPOSED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R057-16 Section 1. Chapter 457 of NAC is hereby amended by adding thereto the following provision: 1. The Division may impose an administrative penalty of $5,000 against any person or organization who is responsible for reporting information on cancer who violates the provisions of NRS 457. 230 and 457.250. 2. The Division shall give notice in the manner set forth in NAC 439.345 before imposing any administrative penalty 3. Any person or organization upon whom the Division imposes an administrative penalty pursuant to this section may appeal the action pursuant to the procedures set forth in NAC 439.300 to 439. 395, inclusive. Section 2. NAC 457.010 is here by amended to read as follows: As used in NAC 457.010 to 457.150, inclusive, unless the context otherwise requires: 1. “Cancer” has the meaning ascribed to it in NRS 457.020. 2. “Division” means the Division of Public and Behavioral Health of the Department of Health and Human Services. 3. “Health care facility” has the meaning ascribed to it in NRS 457.020. 4. “[Malignant neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. [4] “Medical laboratory” has the meaning ascribed to it in NRS 652.060. 5. “Neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. 6. “[Physician] Provider of health care” means a [physician] provider of health care licensed pursuant to chapter [630 or 633] 629.031 of NRS. 7. “Registry” means the office in which the Chief Medical Officer conducts the program for reporting information on cancer and maintains records containing that information. -

WSC 10-11 Conf 7 Layout Master
The Armed Forces Institute of Pathology Department of Veterinary Pathology Conference Coordinator Matthew Wegner, DVM WEDNESDAY SLIDE CONFERENCE 2010-2011 Conference 7 29 September 2010 Conference Moderator: Thomas Lipscomb, DVM, Diplomate ACVP CASE I: 598-10 (AFIP 3165072). sometimes contain many PAS-positive granules which are thought to be phagocytic debris and possibly Signalment: 14-month-old , female, intact, Boxer dog phagocytized organisms that perhaps Boxers and (Canis familiaris). French bulldogs are not able to process due to a genetic lysosomal defect.1 In recent years, the condition has History: Intestine and colon biopsies were submitted been successfully treated with enrofloxacin2 and a new from a patient with chronic diarrhea. report indicates that this treatment correlates with eradication of intramucosal Escherichia coli, and the Gross Pathology: Not reported. few cases that don’t respond have an enrofloxacin- resistant strain of E. coli.3 Histopathologic Description: Colon: The small intestine is normal but the colonic submucosa is greatly The histiocytic influx is reportedly centered in the expanded by swollen, foamy/granular histiocytes that submucosa and into the deep mucosa and may expand occasionally contain a large clear vacuole. A few of through the muscular wall to the serosa and adjacent these histiocytes are in the deep mucosal lamina lymph nodes.1 Mucosal biopsies only may miss the propria as well, between the muscularis mucosa and lesions. Mucosal ulceration progresses with chronicity the crypts. Many scattered small lymphocytes with from superficial erosions to patchy ulcers that stop at plasma cells and neutrophils are also in the submucosa, the submucosa to only patchy intact islands of mucosa. -

Dermatofibrosarcoma Protuberans in a Male Infant
Pediatric Case Reports Dermatofibrosarcoma Protuberans in a Male Infant Leslie Peard, Nicholas G. Cost, and Amanda F. Saltzman Dermtofibrosarcoma protuberans is a rare cutaneous malignancy known to be locally aggressive. It is uncommonly seen in the pediatric population and can be difficult to distinguish from other benign skin lesions. We present a case of dermatofi- brosarcoma protuberans of the penis in a 6-month-old child managed with surgical resection. This case highlights the challenges of diagnosis of genital lesions in children and the complexities of genitourinary reconstruction following surgical resection. UROLOGY 129: 206−209, 2019. © 2018 Elsevier Inc. ermatofibrosarcoma protuberans (DFSP) is a and no frozen section was sent intraoperatively. The rare cutaneous malignancy with reported foreskin was not sent to pathology per institutional D annual incidence of 4.2 per million (0.3 to practice. 1.3 per million in pediatric patients) in the United Pathologic evaluation by a dermatopathologist revealed States. Patients are typically 20-50 years old. DFSP a CD34+ spindle cell neoplasm, favoring DFSP, with most commonly occurs on the trunk, and is very rarely involvement of deep and “lateral” margins (again, the found on the genitalia.1 To our knowledge, only four specimen was not orientated). FISH for the chromosomal cases of penile DFSP have been reported.2-4 The tumor translocation t(17,22) was negative. CT chest obtained is locally aggressive, with few reported cases of metas- for staging was negative for metastasis. After discussion at tasis.1 There is a paucity of data concerning character- multidisciplinary tumor board, options for management istics of disease and treatment strategies with only 2 proposed included Mohs surgery under local anesthesia by published guidelines available to guide management.5,6 dermatology versus wide local excision with frozen section We present a case of DFSP of the penis in an infant, under general anesthesia by urology. -

An Electron Microscope Study of Histiocyte Response to Ascites Tumor Homografts*
An Electron Microscope Study of Histiocyte Response to Ascites Tumor Homografts* L. J. JOURNEYANDD. B. Aiviosf (Experimental Biology Department, Roswell Park Memorial Institute, fÃujfaln.New York) SUMMARY When the ascites forms of the DBA/2 lymphoma L1210 or the C57BL E.L. 4 lymphoma are injected into C3H mice, host histiocytes (macrophages) accumulate and are responsible for the destruction of a large number of tumor cells. Many of the tumor cells, often apparently intact, are ingested. The ingestion process is rapid and depends upon invagination of an area of the histiocyte with simultaneous projection of cytoplasmic fimbriae which complete the encirclement. The earliest change seen in the enclosed cell is shrinkage; digestion of the cell wall and cytoplasmic elements fol lows. Phagocytosis accounts for only a proportion of the cells destroyed by histiocytes. Other cells are probably destroyed when their cell membrane is broken down in an area in contact with a histiocyte, apparently permitting fusion of the two cytoplasms. Weaver and his colleagues (13) observed that cytes and details some of the concomitant changes host cells were frequently found in close associa occurring in the histiocytes themselves. tion with tumor cells and described death of both host and incompatible tumor cell after a period of MATERIALS AND METHODS contact. Gorer (9) found that the predominant In a series of experiments 20 X IO7 cells from host cell in the ascites fluid during tumor rejection rapidly growing ascites populations of the lympho- was the histiocyte. This finding was confirmed, and mas E. L. 4 or L1210 native to C57BL and DBA/ some quantitative measurements were made by 2, respectively, were injected into the peritoneal one of us (1) and later by Weiser and his col cavity of young adult male C3H/He mice. -

Treatment Outcomes of Pediatric Acute Myeloid Leukemia In
children Article Treatment Outcomes of Pediatric Acute Myeloid Leukemia in the Yeungnam Region: A Multicenter Retrospective Study of the Study Alliance of Yeungnam Pediatric Hematology–Oncology (SAYPH) Jae Min Lee 1 , Eu Jeen Yang 2 , Kyung Mi Park 2 , Young-Ho Lee 3, Heewon Chueh 4, Jeong Ok Hah 5, Ji Kyoung Park 6, Jae Young Lim 7, Eun Sil Park 7, Sang Kyu Park 8, Heung Sik Kim 9, Ye Jee Shim 10 , Jeong A. Park 11,12, Eun Jin Choi 13, Kun Soo Lee 14, Ji Yoon Kim 14 and Young Tak Lim 2,* 1 Department of Pediatrics, College of Medicine, Yeungnam University, Daegu 42415, Korea; [email protected] 2 Department of Pediatrics, Pusan National University Children’s Hospital, Pusan National University, School of Medicine, Yangsan 50612, Korea; [email protected] (E.J.Y.); [email protected] (K.M.P.) 3 Department of Pediatrics, Hanyang University College of Medicine, Hanyang University Medical Center, Seoul 04763, Korea; [email protected] 4 Department of Pediatrics, Dong-A University College of Medicine, Busan 49201, Korea; [email protected] 5 Department of Pediatrics, Daegu Fatima Hospital, Daegu 41199, Korea; [email protected] 6 Department of Pediatrics, Inje University College of Medicine, Busan Paik Hospital, Busan 47392, Korea; [email protected] 7 Department of Pediatrics, Gyeongsang National University College of Medicine, Jinju 52727, Korea; [email protected] (J.Y.L.); [email protected] (E.S.P.) Citation: Lee, J.M.; Yang, E.J.; Park, 8 Department of Pediatrics, Ulsan University Hospital, Ulsan 44033, Korea; [email protected] K.M.; Lee, Y.-H.; Chueh, H.; Hah, J.O.; 9 Department of Pediatrics, Keimyung University School of Medicine, Keimyung University Daegu Dongsan Park, J.K.; Lim, J.Y.; Park, E.S.; Park, Hospital, Daegu 41931, Korea; [email protected] S.K.; et al. -
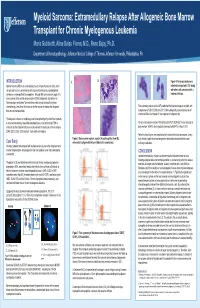
Myeloid Sarcoma
Myeloid Sarcoma: Extramedullary Relapse After Allogeneic Bone Marrow Transplant for Chronic Myelogenous Leukemia Maria Gubbiotti, Alina Dulau Florea, M.D., Renu Bajaj, Ph.D. Department of Hematopathology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA INTRODUCTION A. B. Figure 4: Numerous blasts were Myeloid sarcoma (MS) is an extramedullary tumor of myeloid precursor cells, which detected in the patient’s CSF along can precede or occur concomitantly with acute myeloid leukemia, myelodysplastic with other cells consistent with a syndrome, or myeloproliferative neoplasms. Although MS can involve any organ, it is leukemic infiltrate. more common in the central nervous system (CNS) and gonads, sites known as “pharmacologic sanctuaries” where leukemic cells can survive despite systemic chemotherapy. Less often, this tumor can be the manner of relapse after allogeneic Flow cytometry analysis of the CSF established the blast phenotype as myeloid, with bone marrow transplantation. coexpression of CD13, CD33 and CD117. She subsequently received two cycles of intrathecal ARA-C and repeat LP was negative for malignant cells. The diagnosis is based on morphology and immunophenotype by either flow cytometry or immunohistochemistry of paraffin-embedded tissue, and confirmed by FISH or Reverse transcription real-time PCR detected the P210 BCR-ABL1 fusion transcript in molecular studies. Myeloid sarcomas usually express the leukocyte common antigens bone marrow : 0.064%, which gradually increased to 98.947% in March 2013. CD45, CD13, CD33, CD43 and lack T-cell and B-cell antigens. Patient’s clinical course was complicated with multiple infections (pneumonia, urinary Figure 2: Bone marrow aspirate, regular (A) and magnified view (B), tract infection) septic shock and progressive deterioration despite antibiotics and Case Study demonstrating hypercellularity and blast crisis respectively. -

THE AMERICAN JOURNAL of CANCER a Continuation of the Journal of Cancer Research
THE AMERICAN JOURNAL OF CANCER A Continuation of The Journal of Cancer Research ~ VOLUMEXXXIV DECEMBER,1938 NUMBER4 SYNOVIAL SARCOMAS IN SEROUS BURSAE AND TENDON SHEATHS PROF. LOUIS BERGER, M.D. (From the Pathological Department, HBpital de I'Enfant-Jdsus, and the Anti-Cancer Center of Lava1 University, Quebec) Progress in the knowledge of malignant tumors arising from synovial tissue has been slow. In spite of some recent and valuable contributions, this chapter is far from complete. The reasons for this are threefold: first, the want of knowledge concerning the normal features and nature of synovial tissue, which was long studied in articulations only, although it is common, also, to serous bursae and tendon sheaths; second, the relative-perhaps only apparent-rarity of cases; finally, the lack of precision and even vagueness of the reports in the literature., Most of the older authors, and even some contemporary ones, interested primarily in the clinical or surgical aspects of the question, have been satisfied with a purely topographical diagnosis and have either neglected the histologic aspects of their tumors or described them only briefly and superficially. We have had the opportunity of studying five cases of synovial sarcoma, differing more or less from one another but all originating outside of articu- lations, that is in serous bursae or tendon sheaths, where these tumors are less known, but perhaps easier to study than in the more intricate tissues of the joints. THENORMAL SYNOVIAL TISSUE The prototype of synovial tissue is encountered in the synovial membranes of the joints, but all histologists admit that the lining tissue of the serous bursae and tendon sheaths is homologous with articular synovialis. -

Histiocytic and Dendritic Cell Lesions
1/18/2019 Histiocytic and Dendritic Cell Lesions L. Jeffrey Medeiros, MD MD Anderson Cancer Center Outline 2016 classification of Histiocyte Society Langerhans cell histiocytosis / sarcoma Erdheim-Chester disease Juvenile xanthogranuloma Malignant histiocytosis Histiocytic sarcoma Interdigitating dendritic cell sarcoma Follicular dendritic cell sarcoma Rosai-Dorfman disease Hemophagocytic lymphohistiocytosis Writing Group of the Histiocyte Society 1 1/18/2019 Major Groups of Histiocytic Lesions Group Name L Langerhans-related C Cutaneous and mucocutaneous M Malignant histiocytosis R Rosai-Dorfman disease H Hemophagocytic lymphohistiocytosis Blood 127: 2672, 2016 L Group Langerhans cell histiocytosis Indeterminate cell tumor Erdheim-Chester disease S100 Normal Langerhans cells Langerhans Cell Histiocytosis “Old” Terminology Eosinophilic granuloma Single lesion of bone, LN, or skin Hand-Schuller-Christian disease Lytic lesions of skull, exopthalmos, and diabetes insipidus Sidney Farber Letterer-Siwe disease 1903-1973 Widespread visceral disease involving liver, spleen, bone marrow, and other sites Histiocytosis X Umbrella term proposed by Sidney Farber and then Lichtenstein in 1953 Louis Lichtenstein 1906-1977 2 1/18/2019 Langerhans Cell Histiocytosis Incidence and Disease Distribution Incidence Children: 5-9 x 106 Adults: 1 x 106 Sites of Disease Poor Prognosis Bones 80% Skin 30% Liver Pituitary gland 25% Spleen Liver 15% Bone marrow Spleen 15% Bone Marrow 15% High-risk organs Lymph nodes 10% CNS <5% Blood 127: 2672, 2016 N Engl J Med -

Progress in Understanding the Pathogenesis of Langerhans Cell Histiocytosis: Back to Histiocytosis X?
review Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to Histiocytosis X? Marie-Luise Berres,1,2,3,4 Miriam Merad1,2,3 and Carl E. Allen5,6 1Department of Oncological Sciences, Mount Sinai School of Medicine, 2Tisch Cancer Institute, Mount Sinai School of Medicine, 3Immunology Institute, Mount Sinai School of Medicine, New York, NY, USA, 4Department of Internal Medicine III, University Hospital, RWTH Aachen, Aachen, Germany, 5Texas Children’s Cancer Center, and 6Baylor College of Medicine, Houston, TX, USA Summary Langerhans cell histiocytosis (LCH) is the most common his- tiocytic disorder, arising in approximately five children per Langerhans cell histiocytosis (LCH), the most common million, similar in frequency to paediatric Hodgkin lym- histiocytic disorder, is characterized by the accumulation of phoma and acute myeloid leukaemia (AML) (Guyot-Goubin CD1A+/CD207+ mononuclear phagocytes within granuloma- et al, 2008; Stalemark et al, 2008; Salotti et al, 2009). The tous lesions that can affect nearly all organ systems. Histori- median age of presentation is 30 months, though LCH is cally, LCH has been presumed to arise from transformed or reported in adults in approximately one adult per million, pathologically activated epidermal dendritic cells called Lan- both as unrecognized chronic paediatric disease and de novo gerhans cells. However, new evidence supports a model in disease (Baumgartner et al, 1997). There are occasional which LCH occurs as a consequence of a misguided differen- reports of affected non-twin siblings and multiple cases in tiation programme of myeloid dendritic cell precursors. one family, though it is not clear if this is significantly more Genetic, molecular and functional data implicate activation frequent than one would expect by chance (Arico et al, of the ERK signalling pathway at critical stages in myeloid 2005).