Strelitzia 18 2007.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

News from the CREW
Volume 6 • March 200 News from the CREW lthough 2009 has been a Asteraceae family) in full flower. REW, the Custodians of Areally challenging year with These plants are usually rather C Rare and Endangered the global recession having had inconspicuous and are very hard Wildflowers, is a programme a heavy impact on all of us, it to spot when not flowering, so that involves volunteers from we were very lucky to catch it could not break the strong spir- the public in the monitoring it of CREW. Amidst the great in flower. The CREW team has taken a special interest in the and conservation of South challenges we came up tops genus Marasmodes (we even Africa’s threatened plants. once again, with some excep- have a day in April dedicated to CREW aims to capacitate a tionally great discoveries. the monitoring of this genus) network of volunteers from as they all occur in the lowlands a range of socio-economic Our first great adventure for and are severely threatened. I backgrounds to monitor the year took place in the knew from the herbarium speci- and conserve South Afri- Villiersdorp area. We had to mens that there have not been ca’s threatened plant spe- collect flowering material of any collections of Marasmodes Prismatocarpus lycioides, a data cies. The programme links from the Villiersdorp area and volunteers with their local deficient species in the Campan- was therefore very excited conservation agencies and ulaceae family. We rediscovered about this discovery. As usual, this species in the area in 2008 my first reaction was: ‘It’s a particularly with local land and all we had to go on was a new species!’ but I soon so- stewardship initiatives to en- scrappy nonflowering branch. -

An Overview of Plant Resources and Their Economic Uses in Nigeria
Global Advanced Research Journal of Agricultural Science (ISSN: 2315-5094) Vol. 4(2) pp. 042-067, February, 2015. Available online http://garj.org/garjas/index.htm Copyright © 2015 Global Advanced Research Journals Review An overview of plant resources and their economic uses in Nigeria *Kutama 1, A. S., 1Dangora, I. I., 1Aisha, W. 1Auyo, M. I., 2 Sharif, U. 3Umma, M, and 4Hassan, K. Y. 1Department of Biological Sciences, Federal University, Dutse. P.M.B 7156-Nigeria 2Department of Biological Sciences, College of Arts and Sciences, Kano 3Department of Biology, Kano University of Science &Technology , Wudil . 4 Department of Biology, Sa’adatu Rimi College of Education, Kano Accepted 17 February, 2015 Nigeria is an agrarian country blessed with almost uncountable number of plant species; in water, on land e.t.c. Plants are and remain the indispensable gift of nature given to mankind whose uses were discovered by man even before civilization. This paper reviews some important aspects of plants which include their origin, classification, morphology, as well as economic uses especially in the Nigerian context. It is pertinent therefore that students, researchers as well as readers who are interested in plants would find this paper very educative as it explore majority of plant species and their economic uses in Nigeria. Keyword: plant species, economic uses, taxonomy, morphology, Nigeria. INTRODUCTION Evolution of Plant Over 350 million years ago, the first living organism which mosses, hornworts and liverworts. The bryophytes which resembled a plant appeared. It was the blue - green algae represented the basal group in the evolutionary history of (Cyanophyceae) which lived in the sea and can still be plants may have set the stage for the colonization of the found in many water bodies today. -

080057-13.020.Pdf
' ttolaq orlo{4snquo g Japunselou aas ereq peJeplsuoJlou are slueld ssaql '(€986I 'selBr\\ tnq eloo3) Er.rolcl^pu" eITBrsnVqlnoS rllnoS ,teN ur pezllErnl€uosle sr DUDlqog 'pernbar sr oluts sqt ur Suurnccosetcads aqt;o ,rerAerFcrluc p'elFllsnV Iuetsei&ur spee,{\FtueuuoJl^ue snoues'eluoJeqol IeuualodJql e^EqJo'JrE setcedsouotqog stq El?l eseql.Joemlelcueruou oq1 Sutp:t8el uolsnJuotpeltalel osp suorle8qsolul 'tII€IsnV roqunc (tg6I) frad Jo (986I ) u3arg f,q:og pelunoccelou uxut Bunueserda:aseql Jo o^{l 'Je,re,troq',&eq8te;E urelsod\ ur perncJo ouolqog go salcadsaeJqt tEqt pal€clput SoJCpuE.IoqlnE lslu "l,t\uD eq1,{q suorte,uesqoplerd r?rlullsnvurelsa/(\ uI Je) o4r4srp g'sor:eds puorase 3o oruasa:d aql pep.rore.r(L86I) tu:e4 pue (5861)uear9 q8noqlP'(t66I uuoC't66I u,ti\olg? soru?t'q's9861 'oiruls 'B 'DuDqDg e1oo3 3 a) ullEllsnv ur 8ur:rncco se 3o sercedseuo pezluSocer,{lpraue8 o^€q sasnsusJpu€ sr?Jo[IuPITEISnv luaJeu pJqsllqEtsa,{11nj uaeq lou sEq aBeJspulpezll?JnlEu 'ell€rsnv ,{uuurrog erntulcueuroutf,eJ]oc eql ol peJnpoJlulsdnoJ3 luuld :eq1ofuPtu uI sV ?ll€Jlsnv ur pezrlurnl€ueuroraq e^pq ol eeeouptJlu€JltJV utJqlnosJo e:ouaB3o raqrunue;o a\to st DuolqDg uournpoJtul 'pessnssrposlE sl ErlerlsnVujelse/i\ ulse]f,adsouolqpBpezIIEJntuuol I.,!\PCJe>l(lIV)Dptr|s gpue IMSCJe>lt2qJltlp'g seu"u eqt Jo uorl€crlddrsrrupuerdsaprrn aq; pept,torderu uxq esaql:o1 sdeuruoDnqlJlslp pu€ fol V sr,\^e'IID(J'I)DloL[lqu re.^l,.,'q}re) ( J rurng),solrqr7 gpuu Buerd5( JpuV)r?rt gloo^{S o11olusn?uoB ;pezrusoce:e.IE EXu1 eeJql pu? pe.,'rol^atsI uIlEJlsnVuJe1se16 ut tzttrlqrg 3o salceds ',(]OOd p?zrluJuuueqt ;o .{urouoxrl eqI Z6Z-|8Z :(Z) El DtsltttN eIIEJtsnVuralsol\A ul Sutunc:o (eeeceptJl)DuDlqog;osarceds pezllurnlvu aqtJo /rdel^ej cltuouoxBl V C I'3uIuuD1,14 1g'tqcsdal lreJlsqY 'Err{v qlnos u^\oJ adr] 'clnlllsul 's€1,,luoruorlll] 'LX Btg 3rE^trd In)rrrnlo8IEUontN uinrrrqriH uoldluo). -

Reproductive Ecology of Bird-Pollinated Babiana (Iridaceae): Floral Variation, Mating Patterns and Genetic Diversity
REPRODUCTIVE ECOLOGY OF BIRD-POLLINATED BABIANA (IRIDACEAE): FLORAL VARIATION, MATING PATTERNS AND GENETIC DIVERSITY by Caroli de Waal A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Ecology and Evolutionary Biology University of Toronto © Copyright by Caroli de Waal 2010 REPRODUCTIVE ECOLOGY OF BIRD-POLLINATED BABIANA (IRIDACEAE): FLORAL VARIATION, MATING PATTERNS AND GENETIC DIVERSITY Caroli de Waal Master of Science Department of Ecology and Evolutionary Biology University of Toronto 2010 Abstract Flowering plants possess striking variation in reproductive traits and mating patterns, even among closely related species. In this thesis, I investigate morphological variation, mating and genetic diversity of five taxa of bird-pollinated Babiana (Iridaceae), including two species with specialized bird perches. Field observations in 12 populations demonstrated that sunbirds were the primary pollinators. Babiana ringens exhibited correlated geographic variation in flower and perch size. Controlled field pollinations revealed self-compatibility and low pollen limitation in B. ringens subspecies, and self-incompatibility and chronic pollen limitation in B. hirsuta. Allozyme markers demonstrated moderate to high selfing rates among populations and considerable variation in levels of genetic diversity. In B. ringens there was a positive relation between the geographic and genetic distance of populations. The results of a manipulative field experiment indicated position-dependent herbivory on inflorescences of B. hirsuta and this could play a role in the evolution of specialized bird perches in Babiana. ii Acknowledgments First, I would like to thank my supervisor Spencer Barrett for his wealth of knowledge, his contagious enthusiasm about the natural world, and for pushing me further than I thought I could go. -

Cytological Studies in the Iridaceae
68 Cytologia 23 Cytological Studies in the Iridaceae Edgar Gwynn Department of Biology, Washington College, Chestertown, Maryland, U. S.A. Received October 27, 1957 The Iridaceae are a widespread family of the warm and temperate re gions of the world. There are approximately sixty genera and one thousand species according to Swingle (1946), many of which are or have been cul tivated. Despite their cosmopolitan nature, great numbers and extensive use as ornamentals, apparently less than four hundred species have been investi gated cytologically. Most of those that have been analyzed are in the large, cultivated genera such as Crocus, Gladiolus and. Iris. The present work deals with several South African genera and species apparently unreported hitherto. Material and methods All chromosome counts reported in the present work were obtained from the root tips of germinating seeds. The seeds were supplied by the National Botanic Gardens, Kirstenbosch, Newlands, Cape Province, South Africa. This organization also furnished the generic and specific names in each instance. Before germination, the seeds were soaked, sterilized with mercuric chloride, washed in sterile water and plated out in sterile petri dishes. After some difficulty with germination sufficient root tips were obtained for cytolo gical purposes. The material was fixed in Randolph's modified Navashin fluid for 20-24 hours. Following this treatment the material was embedded in paraffin and cut in serial sections at a thickness of 14 microns. The sections were stained with methyl violet-erythrosin following the schedule of Johansen (1940). These procedures give excellent fixation and very clearly stained chromosomes. Camera lucida drawings were made at a magnification of 1800•~. -
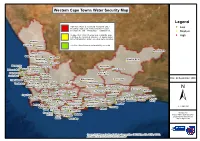
Legend High Risk: Water Demand and Availability Under (! Threat That Requires Urgent Interventions in Resource Low Development and Infrastructure Establishment
Western Cape Towns Water Security Map Legend High Risk: Water Demand and Availability under (! threat that requires urgent interventions in resource Low development and infrastructure establishment. (! Medium Medium Risk: Water Demand and Availability under (! High restriction due to lack of assurance of supply and/or lack of infrastructure and/or exceeding lawful allocation. Lutzville (! Vredendal (! Klawer Low Risk: Water Demand and Availability not at risk (! Murraysburg (! Lamberts Bay (! Graafwater (! (! Wuppertal Clanwilliam (! Beaufort West (! Redelinghuys Citrusdal (! (! Stompneus Eendekuil Merweville Bay Dwarskersbos (! ! (! (! (! Leeu-Gamka Britannia Bay ((!(!Velddrift (! (! Aurora (! Louwville Prince Paternoster (! (! Albert Road (! ! Piketberg Jacobsbaai (! ( (! (! (! (! Hopefield Porterville Saldanha ! Date: 04 September 2020 (! ( Matjies(!fontein Prince Albert Churchhaven Moorreesburg Gouda (! (! ! (! Tulbagh Touwsrivier ( (! Laingsburg Yzerfontein (! R(!iebeek-Wes (!(!Hermon (! (! De Doorns Zoar Darling (! Ceres (! Calitzdorp (! Riebeek-Kasteel Ladismith (! (! ! Dysselsdorp ( (! Oudtshoorn (! Uniondale Paarl (! Worcester Van Wyksdorp (! (! Haarlem (! Montagu (! Volmoed (! Robertson (! (! Cape Town Franschhoek (! Barrydale Karatara (! Ashton (! Ruitersbos ! Plettenberg (! (! (! (! ( Wittedrif Stellenbosch Gena(!dendal Greyton (! Groot Br(!akrivier !Bay(! (!(!(! Suurb(!ra(!ak Heidelberg Riversdale (! (! (! (! (! ( Natures Villiersdorp (! (! B(!randwag Wilderness Knysn(!a (! Bereaville(! (! Albertinia (! Valley ± Grabouw Riviersonderend Slangrivier (! (! (! (! (! Caledon Dana Bay Betty's Bay (! (! (! (! ! Botrivier Klipdale Witsand ( (! (! (! Vlees Bay 1 : 3 000 000 Hawston (! V(!ermo(!nt Napier Malgas Onrus (! Stilbaai He(!rmanus Elim (! Bredasdorp (!(! (! Franskraal Ga(!ns Bay (! Data Source: Arniston Dept of Water and Sanitation Strand Pearly L'Agulhas (!(! Dept of Local Government Beach Struis Bay Dept of Agriculture Source: Esri, Maxar, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AeroGRID, IGN, and the GIS User Community. -

Insights from Microsporogenesis in Asparagales
EVOLUTION & DEVELOPMENT 9:5, 460–471 (2007) Constraints and selection: insights from microsporogenesis in Asparagales Laurent Penet,a,1,Ã Michel Laurin,b Pierre-Henri Gouyon,a,c and Sophie Nadota aLaboratoire Ecologie, Syste´matique et Evolution, Batiment 360, Universite´ Paris-Sud, 91405 Orsay Ce´dex, France bUMR CNRS 7179, Universite´ Paris 6FPierre & Marie Curie, 2 place Jussieu, Case 7077, 75005 Paris, France cMuse´um National d’Histoire Naturelle, De´partement de Syste´matique et Evolution Botanique, 12 rue Buffon, 75005 Paris CP 39, France ÃAuthor for correspondence (email: [email protected]) 1Current address: Department of Biological Sciences, University of Pittsburgh, 4249 Fifth & Ruskin, Pittsburgh, PA 15260, USA. SUMMARY Developmental constraints have been proposed different characteristics of microsporogenesis, only cell to interfere with natural selection in limiting the available wall formation appeared as constrained. We show that set of potential adaptations. Whereas this concept has constraints may also result from biases in the correlated long been debated on theoretical grounds, it has been occurrence of developmental steps (e.g., lack of successive investigated empirically only in a few studies. In this article, cytokinesis when wall formation is centripetal). We document we evaluate the importance of developmental constraints such biases and their potential outcomes, notably the during microsporogenesis (male meiosis in plants), with an establishment of intermediate stages, which allow emphasis on phylogenetic patterns in Asparagales. Different development to bypass such constraints. These insights are developmental constraints were tested by character discussed with regard to potential selection on pollen reshuffling or by simulated distributions. Among the morphology. INTRODUCTION 1991) also hindered tests using the concept (Pigliucci and Kaplan 2000). -

Plant List for Lawn Removal
VERY LOW WATER USE PLANTS Trees * Aesculus californica California buckeye * Cercis occidentalis western redbud * Fremontodendron spp. flannel bush * Pinus abiniana foothill pine * Quercus agrifolia coast live oak * Quercus wislizeni interior live oak Shrubs * Adenostoma fasciciulatum chamise * Arctostaphylos spp. manzanita * Artemesia californica California sagebrush * Ceanothus spp wild lilac * Cercocarpus betuloides mountain mahogany * Amelanchier alnifolia service berry * Dendromecon spp. bush poppy * Heteromeles arbutifolia toyon * Mahonia nevinii Nevin mahonia Perennials * Artemesia tridentata big sagebrush Ballota pseudodictamnus Grecian horehouond * Monardella villosa coyote mint * Nasella needlegrass Penstemon centranthifolius "Scarlet * scarlet bugler penstemon Bugler" * Romneay coulteri Matilija poppy * Salvia apiana white sage * Sisyrinchium bellum blue-eyed grass * Trichostema lanatum woolly blue curls Edibles Olea europaea olive Opunita spp. prickly pear/cholla Cactus and Succulents Cephalocereus spp. old man cactus Echinocactus barrel cactus Graptopetalum spp graptopetalum Bunch Grasses * Bouteloua curtipendula sideoats gramma * Festuca idahoensis Idaho fescue * Leymus condensatus 'Canyon Prince' giant wild rye Bulbs Amaryllis belladona naked lady * Brodiaea spp. brodiaea Colchicum agrippium autumn crocus Muscari macrocarpum grape hyacinth Narcissus spp. daffodil Scilla hughii bluebell Scilla peruviana Peruvian lily Annuals Dimorphotheca spp. African daisy * Eschscholzia californica California poppy Mirabilis jalapa four -

Saldanha Bay Municipality V Britannia Beach Estate Pty
IN THE SUPREME COURT OF APPEAL OF SOUTH AFRICA JUDGMENT CASE NO: 796/11 Reportable In the matter between: SALDANHA BAY MUNICIPALITY APPELLANT and BRITANNIA BEACH ESTATE (PTY) LTD FIRST RESPONDENT BRITANNIA BAY DEVELOPERS (PTY) LTD SECOND RESPONDENT SANDY POINT BEACH PROPERTIES (PTY) LTD THIRD RESPONDENT WEST COAST MIRACLES (PTY) LTD FOURTH RESPONDENT Neutral Citation: Saldanha Bay Municipality v Britannia Beach Estate (Pty) Ltd (796/11) [2012] ZASCA 206 (30 November 2012) Coram: CLOETE and TSHIQI JJA and ERASMUS, SWAIN and MBHA AJJA Heard: 20 November 2012 Delivered: 30 November 2012 2 Summary: Municipal law – local government – the enforceability of conditions in respect of ‘capital contributions’ imposed in terms of s 42 of the Land Use Planning Ordinance 15 of 1985 and the various tariffs underlying such conditions. 3 ______________________________________________________________ ORDER ______________________________________________________________ On appeal from : Western Cape High Court, Cape Town (Cloete AJ sitting as court of first instance): 1 The appeal is upheld with costs, including the costs of two counsel. 2 The order of the court a quo is set aside and substituted with the following: ‘The application is dismissed with costs.’ ______________________________________________________________ JUDGMENT ______________________________________________________________ ERASMUS AJA (CLOETE and TSHIQI JJA and SWAIN and MBHA AJJA concurring): [1] This appeal arises from an order of the Western Cape High Court, Cape Town, declaring the tariff for the calculation of bulk infrastructure development contribution levies, set out in resolutions of the appellant’s council, to be of no force and effect; ordering the appellant to account to the respondents in respect of moneys levied by the appellant and paid by the respondents as contribution levies calculated in accordance with the impugned tariff; and ordering the appellant to pay the respondents’ costs. -

Vol. 4 APPENDIX F-3 Proofs of Submissions Received from I&Aps from Britannica Heights, St Helena Bay and the Petitions
FINAL ENVIRONMENTAL IMPACT ASSESSMENT REPORT Boulders Wind Farm Vol. 4 APPENDIX F-3 Proofs of submissions received from I&APs from Britannica Heights, St Helena Bay and the Petitions September 2019 DRAFT Environmental Impact Assessment Report TABLE OF CONTENTS Proofs of submissions received from I&APs ................................................................ 3 Britannica Heights Residents ......................................................................................... 3 Marx, Matthew ............................................................................................................................. 4 Jordaan, Deborah ....................................................................................................................... 9 Morley Robert ............................................................................................................................ 11 Anne and John Todd ................................................................................................................. 12 Doug Portsmouth ...................................................................................................................... 21 ST Helena Bay Residents ............................................................................................ 24 Smith Dereck ............................................................................................................................. 24 De Kock Colin........................................................................................................................... -

Trip Report Sept
South Africa Birding & Wildlife | Trip Report Sept. 15 – Oct. 1, 2018 | Written by Participant Karen Worcester Photos by Greg Smith With Guides Nick Fordyce, Dalton Gibbs, and Greg Smith, and participants Karen, Dawn, Ralph, Sheri, Robert, Corinne, Andrea, Rensje, Biny, June, Judith, and Wendy Monday, September 17 Arrivals Today was arrival day, with everyone making their way to the Greenwood Guesthouse, which would be our lodging for the next three nights. One of the commonly seen menu items in this part of the world is Butter Curried Chicken, which was served for dinner. Here we met Nick and Dalton, who along with Greg, would be our guides for the next two weeks. All three were energetic, knowledgeable, and charming. Dalton, who managed a reserve system in the Cape areas as his day job, is an encyclopedia for the natural history of this area. He was well versed in botany, could provide geological, geographical, political, and social context with ease, and had a sense of humor as well. He gave an overview of our trip and the extraordinary South African biome we would be exploring. Nick was lively, funny, and kind, and they made a great team. At least 20% of Africa’s plant diversity is in the Cape Town area. There are 319 threatened species and 13 which are already extinct. As small as it is, it is considered the sixth floral kingdom of the world, with 1000 plant species per square kilometer. For example, there are 700 species of Erica (heather) here, while Scotland has only three. Birds are equally diverse, with 950 species and 144 endemics! In this area, the warm waters of the Indian Ocean meet the cold water of the Atlantic, and the climate is “Mediterranean.” In addition, there is great topographic diversity, which causes diversification through isolation. -

Belair National Park
Preliminary Flora List Interim Flora Species List BELAIR NATIONAL PARK (NPWS) Reserve code : NP13 Accepted Species Common name First - Last Record Acacia acinacea Wreath Wattle 01/01/1978 - 22/09/2002 Acacia longifolia var. longifolia * Sallow Wattle 01/01/1978 - 21/05/1986 Acacia melanoxylon Blackwood 01/01/1978 - 21/05/1986 Acacia myrtifolia var. myrtifolia Myrtle Wattle 01/01/1978 - 14/12/1999 Acacia paradoxa Kangaroo Thorn 01/01/1936 - 22/09/2002 Acacia pycnantha Golden Wattle 01/01/1978 - 22/09/2002 Acacia retinodes var. retinodes (hill form) Wirilda 01/01/1936 - 01/01/1936 Acacia spinescens Spiny Wattle 01/01/1936 - 01/01/1936 Acacia verticillata Prickly Moses 01/01/1936 - 01/01/1936 Acaena echinata var. Sheep's Burr 01/01/1936 - 01/01/1936 Acaena novae-zelandiae Biddy-biddy 01/01/1936 - 21/05/1986 Acetosella vulgaris * Sorrel 01/01/1987 - 01/01/1987 Acianthus pusillus Mosquito Orchid 01/01/1936 - 10/07/2000 Acrotriche fasciculiflora Mount Lofty Ground-berry 01/01/1978 - 30/11/2000 Acrotriche serrulata Cushion Ground-berry 01/01/1978 - 30/11/2000 Actinobole uliginosum Flannel Cudweed 01/01/1936 - 01/01/1936 Adiantum aethiopicum Common Maiden-hair 21/05/1986 - 21/05/1986 Agrostis aemula Blown-grass 01/01/1936 - 29/11/2000 Agrostis avenacea var. avenacea Common Blown-grass 21/05/1986 - 21/05/1986 Agrostis capillaris var. capillaris * Brown-top Bent 01/01/1987 - 01/01/1987 Aira cupaniana * Small Hair-grass 01/01/1987 - 01/01/1987 Aira elegantissima ssp. elegantissima * Delicate Hair-grass 29/11/2000 - 29/11/2000 Ajuga australis form B Lesser Bugle 01/01/1936 - 23/09/2000 Allium triquetrum * Three-cornered Garlic 01/01/1987 - 01/01/1987 Allocasuarina muelleriana ssp.