Final Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Telling the Story of the Royal Navy and Its People in the 20Th & 21St
NATIONAL Telling the story of the Royal Navy and its people MUSEUM in the 20th & 21st Centuries OF THE ROYAL NAVY Storehouse 10: New Galleries Project: Exhibition Design Report JULY 2011 NATIONAL MUSEUM OF THE ROYAL NAVY Telling the story of the Royal Navy and its people in the 20th & 21st Centuries Storehouse 10: New Galleries Project: Exhibition Design Report 2 EXHIBITION DESIGN REPORT Contents Contents 1.0 Executive Summary 2.0 Introduction 2.1 Vision, Goal and Mission 2.2 Strategic Context 2.3 Exhibition Objectives 3.0 Design Brief 3.1 Interpretation Strategy 3.2 Target Audiences 3.3 Learning & Participation 3.4 Exhibition Themes 3.5 Special Exhibition Gallery 3.6 Content Detail 4.0 Design Proposals 4.1 Gallery Plan 4.2 Gallery Plan: Visitor Circulation 4.3 Gallery Plan: Media Distribution 4.4 Isometric View 4.5 Finishes 5.0 The Visitor Experience 5.1 Visuals of the Gallery 5.2 Accessibility 6.0 Consultation & Participation EXHIBITION DESIGN REPORT 3 Ratings from HMS Sphinx. In the back row, second left, is Able Seaman Joseph Chidwick who first spotted 6 Africans floating on an upturned tree, after they had escaped from a slave trader on the coast. The Navy’s impact has been felt around the world, in peace as well as war. Here, the ship’s Carpenter on HMS Sphinx sets an enslaved African free following his escape from a slave trader in The slave trader following his capture by a party of Royal Marines and seamen. the Persian Gulf, 1907. 4 EXHIBITION DESIGN REPORT 1.0 Executive Summary 1.0 Executive Summary Enabling people to learn, enjoy and engage with the story of the Royal Navy and understand its impact in making the modern world. -

The Semaphore Circular No 659 the Beating Heart of the RNA May 2016
The Semaphore Circular No 659 The Beating Heart of the RNA May 2016 HMS Mersey alongside in Antigua where she is currently the West Indies Guardship. The photo is courtesy of NCM 6 Shipmate Keith Ridley who was hanging around hoping for an invitation on board!!! This edition is the first on-line version of the Semaphore Circular, unless you have registered with Central Office, it will only be available on the RNA website in the ‘Members Area’ under ‘downloads’ at www.royal-naval-association.co.uk and will be emailed to the branch contact, usually the Hon Sec. 1 Daily Orders 1. April Open Day 2. New Insurance Credits 3. Blonde Joke 4. Service Deferred Pensions 5. Guess Where? 6. Donations 7. HMS Raleigh Open Day 8. Finance Corner 9. RN VC Series – T/Lt Thomas Wilkinson 10. Golf Joke 11. Book Review 12. Operation Neptune – Book Review 13. Aussie Trucker and Emu Joke 14. Legion D’Honneur 15. Covenant Fund 16. Coleman/Ansvar Insurance 17. RNPLS and Yard M/Sweepers 18. Ton Class Association Film 19. What’s the difference Joke 20. Naval Interest Groups Escorted Tours 21. RNRMC Donation 22. B of J - Paterdale 23. Smallie Joke 24. Supporting Seafarers Day Longcast “D’ye hear there” (Branch news) Crossed the Bar – Celebrating a life well lived RNA Benefits Page Shortcast Swinging the Lamp Forms Glossary of terms NCM National Council Member NC National Council AMC Association Management Committee FAC Finance Administration Committee NCh National Chairman NVCh National Vice Chairman NP National President DNP Deputy National President GS General -

THE BULLETIN Volume Seventeen 1873 1 LIVERPOOL NAUTICAL
LIVERPOOL ~AI_ l Tl('AL RESEARCH SOCIETY THE BULLETIN Volume Seventeen 1873 1 LIVERPOOL NAUTICAL RESEARCH SOCIETY BULLETIN The Liverpool Museums \villiam Brown Street Liverpool 3. Hon.Secretary - M.K.Stammers, B.A. Editor -N. R. Pugh There is a pleasure 1n the pathless woods, There is rapture on the lonely shore; There is society, where none intrudes, By the deep sea, and music in its roar. Byron. Vol.XVII No.1 January-i'-iarch 1973 Sl\1-1 J .M. BROVJN - MARINE ARTIST (1873-1965) Fifty years ago a \'/ell known marine artist, Sam J .M. Brown, resided in Belgrave S trcet, Lis card, vlallasey. Of his work, there are still originals and reproductions about, nnd fortunately Liverpool Huseums have some attractive specimens. It happened that the writer once had tea with the family, being in 1925 a school friend of Edwin Brown, the artist's only son. Edwin later became a successful poultry farmer but was not endowed with his father's artistic talents. - 1 - Sam Brown painted for Lamport and Holt, Blue Funnel, Booth, Yeoward Lines etc., in advertising and calendar work. He made several sea voyages to gain atmosphere far his pictures, even to the River Amazon. In local waters his favourite type seemed to be topsail schooners, often used as comparisons to the lordly liners of the above mentioned fleets. About 1930, the Browns moved to NalpD.S, Cheshire, and though Sam exhibited a beautiful picture of swans at the Liver Sketching Club's autumn exhibition one year, no further ship portraiture appeared. In November 1972, I was privileged to attend an exhibition of Murine paintings, on the opening day at the Boydell Galler ies, Castle Street . -

Lead Line Naval Association of Canada Vancouver Island Newsletter
Lead Line Naval Association of Canada Vancouver Island Newsletter July – August 2017 • Volume 32, Issue 4 Crew members from HMCS WINNIPEG currently on POSEIDON CUTLASS, form the number 150 on the flight deck, for Canada's 150th Celebrations on May 11, 2017. Photo by Cpl Carbe Orellana, MARPAC Imaging Services INSIDE THIS ISSUE President's Message ............................................2 Ship uniquely marks Canada 150 .........................8 NAC-VI AGM news .............................................3 Polar Flag to fly again .......................................10 NOTC officers learn about diplomacy ................ 4-5 Keel-laying ceremony for HMCS Margaret Brooke ..12 Veteran's Corner .................................................6 Brodeurs gift large collection to museum ......... 14-15 PRESIDENT'S MESSAGE MEMBERSHIP AND EVENT UPDATE Fellow members, last year, we will be taking Canada. As I write this, it feels proxies back with us. To This fall, as well, we will be like summer has finally ar- this end, we will be gather- conducting a members sur- rived. It seems to have taken ing your proxies starting in vey regarding our luncheons awhile. August and working into and speaker program. Our We have had a busy spring September. Once the list of goal is to make sure our plans with a number of new initia- Director candidates is final- stay relevant to the needs and tives. The most recent was ized and the agenda for the wishes of the members. This an excellent presentation by meeting established, I will will also include the possibil- David Collins to the MARS send an email to you all with ity of special member events Trainees at Fleet School the details. -

How Slaves Used Northern Seaports' Maritime Industry to Escape And
Eastern Illinois University The Keep Faculty Research & Creative Activity History May 2008 Ports of Slavery, Ports of Freedom: How Slaves Used Northern Seaports’ Maritime Industry To Escape and Create Trans-Atlantic Identities, 1713-1783 Charles Foy Eastern Illinois University, [email protected] Follow this and additional works at: http://thekeep.eiu.edu/history_fac Part of the United States History Commons Recommended Citation Foy, Charles, "Ports of Slavery, Ports of Freedom: How Slaves Used Northern Seaports’ Maritime Industry To Escape and Create Trans-Atlantic Identities, 1713-1783" (2008). Faculty Research & Creative Activity. 7. http://thekeep.eiu.edu/history_fac/7 This Article is brought to you for free and open access by the History at The Keep. It has been accepted for inclusion in Faculty Research & Creative Activity by an authorized administrator of The Keep. For more information, please contact [email protected]. © Charles R. Foy 2008 All rights reserved PORTS OF SLAVERY, PORTS OF FREEDOM: HOW SLAVES USED NORTHERN SEAPORTS’ MARITIME INDUSTRY TO ESCAPE AND CREATE TRANS-ATLANTIC IDENTITIES, 1713-1783 By Charles R. Foy A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Graduate Program in History written under the direction of Dr. Jan Ellen Lewis and approved by ______________________ ______________________ ______________________ ______________________ ______________________ New Brunswick, New Jersey May, 2008 ABSTRACT OF THE DISSERTATION PORTS OF SLAVERY, PORTS OF FREEDOM: HOW SLAVES USED NORTHERN SEAPORTS’ MARITIME INDUSTRY TO ESCAPE AND CREATE TRANS-ATLANTIC IDENTIES, 1713-1783 By Charles R. Foy This dissertAtion exAmines and reconstructs the lives of fugitive slAves who used the mAritime industries in New York, PhilAdelphiA and Newport to achieve freedom. -

1 ' W ' WADSWORTH, James Bruce, Electrical Lieutenant
' W ' WADSWORTH, James Bruce, Electrical Lieutenant - Member - Order of the British Empire (MBE) - RCN - Awarded as per London Gazette of 11 December 1945 (no Canada Gazette). Home: Ste. Hyacinthe, Quebec. WADSWORTH. James Bruce, 0-75310, Lt(El) [1.7.42] RCN MBE~[11.12.45] Lt(L) [1.7.42] HMCS STADACONA(D/S) for Elect/School, (18.1.46-?) RCNB Esquimalt, (15.12.47-?) HMCS ROCKCLIFFE(D/S)(J355) (25.8.49-?) LCdr(L) [1.7.50] RCNB Esquimalt, Elect/Trg/Centre OIC, (15.8.50-?) "For distinguished service during the war in Europe." * * * * * WADSWORTH, Rein Boulton, Lieutenant-Commander - Distinguished Service Cross (DSC) - RCNVR / at Salerno - Awarded as per Canada Gazette of 24 June 1944 and London Gazette of 23 May 1944. Home: Toronto, Ontario. He left for England with the first group of officers from HMCS York (Naval Reserve Division) as a Sub-Lieutenant in 1940. Commanding Officer of LST 319 ("Philadelphia") during WW2 at the landing at Salerno, Italy, for which he received the Distinguished Service Cross. WADSWORTH. Rein Boulton, RCNVR Company Toronto [18.3.28] RCNVR S/Lt [18.3.29] Lt(Temp) [24.7.40] LCdr(Temp) [1.7.43] DSC~[24.6.44] Cdr(Temp) Retired [29.9.44] "For good service in attack on Salerno." * * * * * WAGG, Frank, Chief Petty Officer (A-5386) - Mention in Despatches - RCNR - Awarded as per Canada Gazette of 16 June 1945 and London Gazette of 14 June 1945. Home: Gore Bay, Ontario. WAGG. Frank, A-5386, CPO, MID~[16.6.45] "Chief Petty Officer Wagg set a good example by his cheerfulness during the strenuous period of hours at the wheel. -

Hk ^^^K^^L V^F ^H
my • .-V ^% Hk ^^^k^^l v^f ^H t^^^^^^P ^ fe£& -# \ * • 4 *S5** *^' -^. i - ;i»» ..... CONTENTS "KEMBLA II JULY. 1953. COPPER, BRASS AND EDITORIAL: M.V. '•IXJNTROON"— 10.500 ion. OTHER NON-FERROUS British Admiralty's New Test Houie For Gat Turbine Engines * Liaison Between Royal and Merchant Navies 5 MELBOURNE WIRE CABLES & TUBES Oil and Our Destiny . 5 STEAMSHIP Centenary c: R.N. Continuous Service Engagement S CO. LTD. METAL MANUFACTURES LTD. ARTICLES: Hud Office: 31 KING ST.. MELBOURNE PORT KEMBLA. N.S.W. Aircraft Carriers are Indispemible • 6 Searchers of the Sea Depths 8 SELLING AGENTS The Coronation Naval Review . II fwitll DiMffhufPrj in ill Suin New Base in U.K. for Minesweepers and Patrol Boats 13 MANAGING AGENTS FOR Boyd Trophy Presentation Ceremony 13 HOBSONS BAY DOCK AND TVBbS X BRASS WIRl VntE a. CABLES Completion of Shaw Savill M.V. "Cymric'' 23 ENGINEERING CO. PTY. LTD. KNOX SCHLAPP PTY. LTD. BRITISH INSULATED Combined Indian Ocean Exercises 27 Works: Williamstown, Victoria CALLENDER'S CABLES Cadets Visit Nelon s Dockyard . .. 27 and Coronation Carrier Returning Home , ... 29 Collins House, Melbourne LTD. HODGE ENGINEERING CO. 84 William St., Melbourne PTY. LTD. Kembla Building, Sydney 44 Margaret St., Sydney. FEATURES: Works: Sussex St., Sydney. News of the World's Navies IB SHIP REPAIRERS. ETC. Maritime News of the World 19 J — Personal Paragraphs 22 Sea Oddities 24 Speaking of Ships 26 Book Reviews . - 28 ZINC ASSOCIATIONS. CLUBS: ~it is a Si-Navel Men's Association of Australia 30 Without this essential metal there would be pleasure NO GALVANIZED PRODUCTS and Published bv The Navy League, Royal Exchange Building. -

RR Indepth Issue 19 Autumn 2013.Indb
issue 19 2013 INNOVATIVE LNG FUELLED VESSEL NAVAL SHIP DELIVERIES CONTINUE DESIGNS ALUMINIUM WATERJETS FOR INTRODUCED EFFICIENT PROPULSION ISSUE 19 CONTENTS NEWS News and future events 04 TECHNOLOGY Focusing on innovative naval ship and systems design 08 New applications for permanent magnet motor technology 12 Introducing the MT7 marine gas turbine 14 Promas+nozzle for efficient high bollard pull applications 16 10 PROJECT FOCUS Queen Elizabeth is taking shape 18 18 UPDATES Safely transferring five-tonne loads at sea 22 Aluminium waterjets deliver on performance and reliability 26 LNG power for cruise ferry and Environship 28 Walk to work offshore 30 CUSTOMER SUPPORT AND SERVICE Honing the skills that help locate offshore oil and gas 32 Upgrading MY O’Mega for a new level of comfort 36 CONTACTS 38 30 Front cover: New naval ship designs are now being developed by Rolls-Royce for a range of applications that include patrol craft and logistics vessels. See page 8. Opinions expressed may not necessarily represent the views of Rolls-Royce or the editorial team. The publishers cannot accept liability for errors or omissions. All photographs © Rolls-Royce plc unless otherwise stated. In which case copyright owned by photographer/organisation. EDITOR: Andrew Rice DESIGNED BY: Connect Communications CONTRIBUTORS: RW - Richard White | RS - Richard Scott | MB - Martin Brodie | GA - Gary Atkins | AR - Andrew Rice Printed in the UK. 37 If your details have changed or if you wish to receive a regular complimentary copy of In-depth please email us at: [email protected] © Rolls-Royce plc 2013 The information in this document is the property of Rolls-Royce plc and may not be copied, communicated to a third party, or used for any purpose other than that for which it is supplied, without the express written consent of Rolls-Royce plc. -

The S.S. Gaspesia (Left) and S.S. North Shore (Right) at Montreal's Victoria
CHAPTER 3 The s.s. Gaspesia (left) and s.s. North Shore (right) at Montreal’s Victoria Pier c. 1922 THE CLARKE STEAMSHIP COMPANY: FORMATIVE YEARS During its formative years, although they had successfully been able to export their woodpulp in chartered ships, the Clarke enterprises on the North Shore had most recently suffered from poor inbound transport services. Several companies had tried to establish subsidized steamship services between Quebec and the North Shore, but the fact that they had met with shipwreck and failure meant that the contract had changed hands quite often, especially since the outbreak of war in 1914. The area did of course have its problems. A sparse population scattered over a coastline of nearly 800 miles between Quebec and the Strait of Belle Isle at Blanc-Sablon, a lack of adequate harbour and docking facilities, and the many shoals of the river altogether presented a formidable barrier to operating a regular and profitable steamship line on the Gulf of St Lawrence. Clarke City was less than half way to the Strait of Belle Isle and the whole of the Quebec North Shore around 1920 had a total population of only about 15,000 from Tadoussac to Blanc-Sablon, of which one-third was below Clarke City. Since the South Shore to Gaspé and Prince Edward Island services had sometimes relied upon the same steamship services, a similar story could be told there. Not only had this region lost the services of the Quebec Steamship Co when the Cascapedia was withdrawn from the Gaspé Coast at the end of 1914 and then from Prince Edward Island and Nova Scotia in 1917, but it had also suffered the loss of the Lady of Gaspé. -

RCNR / HMCS Fundy • Awarded As Per Canada Gazette of 5 June 1943 and London Gazette of 2 June 1943
' R ' RAINE, John Buxton, Lieutenant - Mention in Despatches - RCNR / HMCS Fundy • Awarded as per Canada Gazette of 5 June 1943 and London Gazette of 2 June 1943. Home: Peterborough, Ontario. Commanding Officer of HMCS Fundy (I) (Fundy Class Minesweeper - J88) from 29 October 1941 to 9 March 1942 (Rank was Mate). Commanding Officer of HMCS Wasaga (Bangor Class Minesweeper - J162) from 18 June 1943 to 22 December 1943. Only Commanding Officer of HMCS Peterborough (Revised Flower Class Corvette Increased Endurance - K342) from 1 June 1944 to 19 July 1945 (rank of Lieutenant). RAINE. John Buxton, 0-60830, Mate(Temp) [5.4.40] RCNR HMCS ARRAS (J15) 357/17, tr, (5.5.40-?) HMCS FUNDY (J88) m/s, (21.2.41-?) HMCS FUNDY (J88) m/s, CO, (29.10.41-9.3.42) HMCS WASAGA( J162) m/s, CO, (10.3.42-13.3.43) Lt(Temp) [6.4.42] MID~[5.6.43] HMCS WASAGA (J162) m/s, CO, (18.6.43-22.12.43) Lt(Temp) [6.4.41] HMCS PETERBOROUGH (K342) Cofm, CO, stand by (20.3.44-30.5.44) HMCS PETERBOROUGH(K342) Cofm, CO, (1.6.44-19.7.45) Demobilized [20.10.45] "This Officer has rendered consistently good service as Commanding Officer of one of His Majesty's Canadian Minesweepers (HMCS Fundy) in the North Atlantic. He displayed outstanding seamanship and devotion to duty in very heavy weather whilst assisting in the salving of one of His Majesty's Ships." * * * * * RAINES, Frederick Arthur, Shipwright Lieutenant - Member - Order of the British Empire (MBE) - RCN / Senior Shipwright Officer of HMCS Avalon (St. -
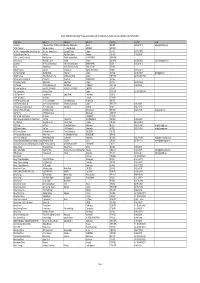
DIUS Register Final Version
Register of Education and Training Providers as last maintained by the Department of Innovation, Universities and Skills on the 30 March 2009 College Name Address 1 Address 2 Address 3 Postcode Telephone Email 12 training 1 Sherwood Place, 153 Sherwood DrivBletchley, Milton Keynes Bucks MK3 6RT 0845 605 1212 [email protected] 16 Plus Team Ltd Oakridge Chambers 1 - 3 Oakridge Road BROMLEY BR1 5QW 1st Choice Training and Assessment Centre Ltd 8th Floor, Hannibal House Elephant & Castle London SE1 6TE 020 7277 0979 1st Great Western Train Co 1st Floor High Street Station Swansea SA1 1NU 01792 632238 2 Sisters Premier Division Ltd Ram Boulevard Foxhills Industrial Estate SCUNTHORPE DN15 8QW 21st Century I.T 78a Rushey Green Catford London SE6 4HW 020 8690 0252 [email protected] 2C Limited 7th Floor Lombard House 145 Great Charles Street BIRMINGHAM B3 3LP 0121 200 1112 2C Ltd Victoria House 287a Duke Street, Fenton Stoke on Trent ST4 3NT 2nd City Academy City Gate 25 Moat Lane Digbeth, Birmingham B5 5BD 0121 622 2212 2XL Training Limited 662 High Road Tottenham London N17 0AB 020 8493 0047 [email protected] 360 GSP College Trident Business Centre 89 Bickersteth Road London SW17 9SH 020 8672 4151 / 084 3E'S Enterprises (Trading) Ltd Po Box 1017 Cooks Lane BIRMINGHAM B37 6NZ 5 E College of London Selby Centre Selby Road London N17 8JL 020 8885 3456 5Cs Training 1st Floor Kingston Court Walsall Road CANNOCK WS11 0HG 01543 572241 6S Consulting Limited c/o 67 OCEAN WHARF 60 WESTFERRY ROAD LONDON E14 8JS 7city Learning Ltd 4 Chiswell -

LINOLEUMS FIELD SEEDS Hr 10
aili) THERE ARE OTHERS ! Bat wo doubt tf they «re QriTTS n« »ihW! es our Tea*. ami w* »r«* ik>m1- Household Coal tlroly i vrtàtn you will have to pay a rroHt don I morn for thorn. • Wrect Imprr'ing lea MdCoftoC Ce. S6.C0 Cor. Dougina au«l Johnaon Street a. Phone, hub________Victoria, b. C. MALL O WALKER,. *• CevenuÉent St 'Phone, Si nVOL. 85 VICTORIA» B. C„ TIIVH8 0AT» MAY 1, 1902. Nt>. ft. 2 GOOD BUYS THh U. S. CHINESE EXCLUSION BILL A new 5 rotxmad- eettago, *««i*c foundation* 2 M*. garden planted. orchard, ettr. ■i BIC STAMPEDE ■II for ................................................................................................................................... «1,100 A beautiful 6 roomed cottage, cor. lot, near Park- Van he bought ou ea»> term*. TO LET T roomed botme, pandora Av#................. Hrt 4 roomed cottage, N. Vhathum St............. %Hr’> 10 6 roomed hvuav, Whittaker Are....... 114 FuruUhed house, Bellott St.......................... ! Canes and! v iwaaisvmm ; --------------!— : mqney to loan at low rates t CONCESSION THROWN P. C. MacGregor & Co.. Agents, 2 View- Street. m - OPEN FOR RE LOCATION lç!Î!P°K'r ‘«.4 J* «4 *4 .4 ,4 ,4 ,4 .4 .4 .4 4 4 4 4 4 4 t4 ,4 vtf .4 „4 "4 .4 4 tf tf tf tf tf 4 tf tf 4 ! Umbrellas ! * J* -* '* <4 ,4 _4 .tf .4 vtf .4 M * ,4 .4 jl „tf j« .tf vtf .tf .tf Mjàjt'j» > ,4 Itf > 'tf ii Litigation Is Likely to Follow—Wash- Up in Progress-The Export Gold, Silverand G. & W. Tax. 1884 SPECIAL. Fancy Heads. <«rwcui it, nww ».