Dissection of the Molecular Targets and Signaling Pathways of Guzhi Zengsheng Zhitongwan Based on the Analysis of Serum Proteomi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification and Partial Characterization of a Family of Putative Palmitoyltransferases in Dictyostelium Discoideum Brent Elliot Wells
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library 2003 Identification and Partial Characterization of a Family of Putative Palmitoyltransferases in Dictyostelium Discoideum Brent Elliot Wells Follow this and additional works at: http://digitalcommons.library.umaine.edu/etd Part of the Biochemistry Commons Recommended Citation Wells, Brent Elliot, "Identification and Partial Characterization of a Family of Putative Palmitoyltransferases in Dictyostelium Discoideum" (2003). Electronic Theses and Dissertations. 301. http://digitalcommons.library.umaine.edu/etd/301 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. IDENTIFICATION AND PARTIAL CHARACTERIZATION OF A FAMILY OF PUTATIVE PALMITOYLTRANSFERASES IN DICTYOSTELIUM DISCOIDEUM BY Brent Elliott Wells B.S. University of Utah, 1994 A THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science (in Biochemistry) The Graduate School The University of Maine December, 2003 Advisory Committee: Robert E. Gundersen, Associate Professor of Biochemistry, Advisor Keith W. Hutchison, Professor of Biochemistry Mary Rumpho-Kennedy, Professor of Biochemistry LIBRARY RIGHTS STATEMENT In presenting this thesis in partial fulfillment of the requirements for an advanced degree at The University of Maine, I agree that the Library shall make it freely available for inspection. I further agree that permission for "fair use" copying of this thesis for scholarly purposes may be granted by the Librarian. It is understood that any copying or publication of this thesis for financial gain shall not be allowed without my written permission. -

Type of the Paper (Article
Supplementary Material A Proteomics Study on the Mechanism of Nutmeg-induced Hepatotoxicity Wei Xia 1, †, Zhipeng Cao 1, †, Xiaoyu Zhang 1 and Lina Gao 1,* 1 School of Forensic Medicine, China Medical University, Shenyang 110122, P. R. China; lessen- [email protected] (W.X.); [email protected] (Z.C.); [email protected] (X.Z.) † The authors contributed equally to this work. * Correspondence: [email protected] Figure S1. Table S1. Peptide fraction separation liquid chromatography elution gradient table. Time (min) Flow rate (mL/min) Mobile phase A (%) Mobile phase B (%) 0 1 97 3 10 1 95 5 30 1 80 20 48 1 60 40 50 1 50 50 53 1 30 70 54 1 0 100 1 Table 2. Liquid chromatography elution gradient table. Time (min) Flow rate (nL/min) Mobile phase A (%) Mobile phase B (%) 0 600 94 6 2 600 83 17 82 600 60 40 84 600 50 50 85 600 45 55 90 600 0 100 Table S3. The analysis parameter of Proteome Discoverer 2.2. Item Value Type of Quantification Reporter Quantification (TMT) Enzyme Trypsin Max.Missed Cleavage Sites 2 Precursor Mass Tolerance 10 ppm Fragment Mass Tolerance 0.02 Da Dynamic Modification Oxidation/+15.995 Da (M) and TMT /+229.163 Da (K,Y) N-Terminal Modification Acetyl/+42.011 Da (N-Terminal) and TMT /+229.163 Da (N-Terminal) Static Modification Carbamidomethyl/+57.021 Da (C) 2 Table S4. The DEPs between the low-dose group and the control group. Protein Gene Fold Change P value Trend mRNA H2-K1 0.380 0.010 down Glutamine synthetase 0.426 0.022 down Annexin Anxa6 0.447 0.032 down mRNA H2-D1 0.467 0.002 down Ribokinase Rbks 0.487 0.000 -

Inorganic Pyrophosphatase Is a Component of the Drosophila Nucleosome Remodeling Factor Complex
Downloaded from genesdev.cshlp.org on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Inorganic pyrophosphatase is a component of the Drosophila nucleosome remodeling factor complex David A. Gdula, Raphael Sandaltzopoulos, Toshio Tsukiyama,1 Vincent Ossipow, and Carl Wu2 Laboratory of Molecular Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892-4255 USA The Drosophila nucleosome remodeling factor (NURF) is a protein complex consisting of four polypeptides that facilitates the perturbation of chromatin structure in vitro in an ATP-dependent manner. The 140-kD NURF subunit, imitation switch (ISWI), is related to the SWI2/SNF2 ATPase. Another subunit, NURF-55, is a 55-kD WD repeat protein homologous to the human retinoblastoma-associated protein RbAp48. Here, we report the cloning and characterization of the smallest (38 kD) component of NURF. NURF-38 is strikingly homologous to known inorganic pyrophosphatases. Both recombinant NURF-38 alone and the purified NURF complex are shown to have inorganic pyrophosphatase activity. Inhibition of the pyrophosphatase activity of NURF with sodium fluoride has no significant effect on chromatin remodeling, indicating that these two activities may be biochemically uncoupled. Our results suggest that NURF-38 may serve a structural or regulatory role in the complex. Alternatively, because accumulation of unhydrolyzed pyrophosphate during nucleotide incorporation inhibits polymerization, NURF may also have been adapted to deliver pyrophosphatase -
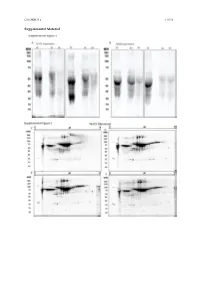
Type of the Paper (Article
Cells 2020, 9, x 1 of 19 Supplemental Material Cells 2020, 9, x 2 of 19 Figure S1. Secretome enrichment: protocol optimization. 1D SDS-PAGE documentation of washing steps: Culture medium was substituted with FCS-free medium, which was changed every 2 h. The supernatants were then collected, and the proteins isolated and separated in 1D SDS-PAGE ((A) TK173 and (B) TK188). Proteins were stained with Flamingo fluorescent gel stain. Two-dimensional pattern of the proteins isolated from supernatant of TK173, (C) 2 h, (D) 4 h, (E) 6 h, and (F) 8 h after changing to FCS-free medium. (G) Cell secretome collected 24 h after elimination of the contaminating FCS-proteins with different washing steps. Proteins were stained with Flamingo fluorescent gel stain. Cells 2020, 9, x 3 of 19 Figure S2. 2-DE reference maps of secretomes; 150 μg proteins were loaded on an 11 cm IPG strip with a linear pH gradient PI 5–8 for IEF; 12% SDS-polyacrylamide gels were used for the second dimension. Proteins were stained with Flamingo fluorescent gel stain. Identified spots were assigned a number corresponding to that in their table. 2-DE maps from secretome of (A) TK173 control and (B) TGFβ1- treated ones. The 2-DE patterns revealed an alteration of secretome in stimulated TK173. Secretome patterns from TK173 treated with (C) ANG II and (D) PDGF. Cells 2020, 9, x 4 of 19 A Figure S3. Classification of the differentially expressed proteins upon ANG II, TGFβ1, or PDGF treatment in TK173. (A) Bar charts of the cellular component analyzed by STRAP biological function analysis in which the identified proteins from all treatments in both cell types are involved. -

The Structural Basis for Pyrophosphatase Catalysis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Research Article 1491 The structural basis for pyrophosphatase catalysis Pirkko Heikinheimo1,2, Jukka Lehtonen1,2, Alexander Baykov3, Reijo Lahti2, Barry S Cooperman4* and Adrian Goldman1,2* Background: Soluble inorganic pyrophosphatase (PPase), an essential Addresses: 1Turku Centre for Biotechnology, PO enzyme central to phosphorus metabolism, catalyzes the hydrolysis of the phos- Box 123, FIN-20521 Turku, Finland, 2Department phoanhydride bond in inorganic pyrophosphate. Catalysis requires divalent of Biochemistry, University of Turku, FIN-20014 Turku, Finland, 3A.N. Belozersky Institute of metal ions which affect the apparent pKas of the essential general acid and Physico-Chemical Biology, Moscow State base on the enzyme, and the pKa of the substrate. Three to five metal ions are University, Moscow 119899, Russia and required for maximal activity, depending on pH and enzyme source. A detailed 4Department of Chemistry, University of understanding of catalysis would aid both in understanding the nature of biolog- Pennsylvania, Philadelphia, PA 19104, USA. ical mechanisms of phosphoryl transfer, and in understanding the role of diva- *Corresponding authors. lent cations. Without a high-resolution complex structure such a model has E-mail: [email protected] previously been unobtainable. E-mail: [email protected] Key words: mechanism, phosphoanhydride Results: We report the first two high-resolution structures of yeast PPase, at hydrolysis, phosphoryl transfer, pyrophosphatase, 2.2 and 2.0 Å resolution with R factors of around 17 %. One structure contains refinement, structure the two activating metal ions; the other, the product (MnPi)2 as well. -

Isomer-Specific Comparisons of the Hydrolysis of Synthetic Pyrethroids and Their Fluorogenic Analogues by Esterases from The
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Pesticide Biochemistry and Physiology 121 (2015) 102–106 Contents lists available at ScienceDirect Pesticide Biochemistry and Physiology journal homepage: www.elsevier.com/locate/pest Isomer-specific comparisons of the hydrolysis of synthetic pyrethroids and their fluorogenic analogues by esterases from the cotton bollworm Helicoverpa armigera G. Yuan a,b, Y. Li c, C.A. Farnsworth d,e,f, C.W. Coppin d, A.L. Devonshire d, C. Scott d, R.J. Russell d, Y. Wu b, J.G. Oakeshott d,* a Key laboratory of Entomology and Pest Control Engineering, College of Plant Protection, Southwest University, Chongqing 400716, China b Department of Entomology, College of Plant Protection, Key Laboratory of Monitoring and Management of Crop Diseases and Pest Insects (Ministry of Agriculture), Nanjing Agricultural University, Nanjing 210095, China c Research and Development Centre of Biorational Pesticides, Northwest Agriculture and Forestry University, Yangling, China d CSIRO Land & Water Flagship, ACT, Australia e School of Biological Sciences, Australian National University, ACT, Australia f Cotton Catchment Communities CRC, Narrabri, NSW, Australia ARTICLE INFO ABSTRACT Article history: The low aqueous solubility and chiral complexity of synthetic pyrethroids, together with large differ- Received 31 October 2014 ences between isomers in their insecticidal potency, have hindered the development of meaningful assays Accepted 10 December 2014 of their metabolism and metabolic resistance to them. To overcome these problems, Shan and Hammock Available online 16 December 2014 (2001) [7] therefore developed fluorogenic and more water-soluble analogues of all the individual isomers of the commonly used Type 2 pyrethroids, cypermethrin and fenvalerate. -

In Vivo Mapping of a GPCR Interactome Using Knockin Mice
In vivo mapping of a GPCR interactome using knockin mice Jade Degrandmaisona,b,c,d,e,1, Khaled Abdallahb,c,d,1, Véronique Blaisb,c,d, Samuel Géniera,c,d, Marie-Pier Lalumièrea,c,d, Francis Bergeronb,c,d,e, Catherine M. Cahillf,g,h, Jim Boulterf,g,h, Christine L. Lavoieb,c,d,i, Jean-Luc Parenta,c,d,i,2, and Louis Gendronb,c,d,i,j,k,2 aDépartement de Médecine, Université de Sherbrooke, Sherbrooke, QC J1H 5N4, Canada; bDépartement de Pharmacologie–Physiologie, Université de Sherbrooke, Sherbrooke, QC J1H 5N4, Canada; cFaculté de Médecine et des Sciences de la Santé, Université de Sherbrooke, Sherbrooke, QC J1H 5N4, Canada; dCentre de Recherche du Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, QC J1H 5N4, Canada; eQuebec Network of Junior Pain Investigators, Sherbrooke, QC J1H 5N4, Canada; fDepartment of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, CA 90095; gSemel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, CA 90095; hShirley and Stefan Hatos Center for Neuropharmacology, University of California, Los Angeles, CA 90095; iInstitut de Pharmacologie de Sherbrooke, Sherbrooke, QC J1H 5N4, Canada; jDépartement d’Anesthésiologie, Université de Sherbrooke, Sherbrooke, QC J1H 5N4, Canada; and kQuebec Pain Research Network, Sherbrooke, QC J1H 5N4, Canada Edited by Brian K. Kobilka, Stanford University School of Medicine, Stanford, CA, and approved April 9, 2020 (received for review October 16, 2019) With over 30% of current medications targeting this family of attenuates pain hypersensitivities in several chronic pain models proteins, G-protein–coupled receptors (GPCRs) remain invaluable including neuropathic, inflammatory, diabetic, and cancer pain therapeutic targets. -

Hydrolysis of Individual Isomers of Fluorogenic Pyrethroid Analogs by Mutant Carboxylesterases from Lucilia Cuprina
ARTICLE IN PRESS Insect Biochemistry and Molecular Biology Insect Biochemistry and Molecular Biology 37 (2007) 891–902 www.elsevier.com/locate/ibmb Hydrolysis of individual isomers of fluorogenic pyrethroid analogs by mutant carboxylesterases from Lucilia cuprina A.L. Devonshirea, R. Heidaria, H.Z. Huangb, B.D. Hammockb, R.J. Russella, J.G. Oakeshotta,Ã aCSIRO Entomology, GPO Box 1700, Canberra, ACT, 2601, Australia bDepartment of Entomology and Cancer Research Center, University of California, Davis, CA 95616, USA Received 28 November 2006; received in revised form 16 April 2007; accepted 17 April 2007 Abstract We previously showed that wild-type E3 carboxylesterase of Lucilia cuprina has high activity against Type 1 pyrethroids but much less for the bulkier, a-cyano containing Type 2 pyrethroids. Both Types have at least two optical centres and, at least for the Type 1 compounds, we found that wild-type E3 strongly prefers the less insecticidal configurations of the acyl group. However, substitutions to smaller residues at two sites in the acyl pocket of the enzyme substantially increased overall activity, particularly for the more insecticidal isomers. Here we extend these analyses to Type 2 pyrethroids by using fluorogenic analogs of all the diastereomers of cypermethrin and fenvalerate. Wild-type E3 hydrolysed some of these appreciably, but, again, not those corresponding to the most insecticidal isomers. Mutations in the leaving group pocket or oxyanion hole were again generally neutral or deleterious. However, the two sets of mutants in the acyl pocket again improved activity for the more insecticidal acyl group arrangements as well as for the more insecticidal configuration of the cyano moiety on the leaving group. -

ALLOSTERIC REGULATION of GS on AGONIST, ANTAGONIST and INVERSE AGONIST BINDING to the Β2ar by Gisselle A. Vélez Ruiz a Dissert
ALLOSTERIC REGULATION OF GS ON AGONIST, ANTAGONIST AND INVERSE AGONIST BINDING TO THE β2AR by Gisselle A. Vélez Ruiz A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Cellular and Molecular Biology) in the University of Michigan 2011 Doctoral Committee: Associate Professor Roger K. Sunahara, Chair Professor Richard R. Neubig Professor Michael D. Uhler Associate Professor Martin Myers Associate Professor John J.G. Tesmer To my family especially my mom and siblings, best friends: Grisel and Nelly, and to my boyfriend Osvaldo. Your constant love, support and eternal encouragement gave me the strength and motivation to accomplish my goals. ii ACKNOWLEDGEMENTS I would like to thank my mentor Roger Sunahara for all his support and endless enthusiasm. His support and patience allowed me to become a great researcher. I would also like to thank all the members of the Sunahara lab, past and present, especially Dr. Matthew Whorton and Dr. Adam Kuszak. Thank you for all your help and motivation especially when science was not on my side. Thank you for the great science but most importantly for the laughs; not matter how bad my day was they always found a way to make me laugh and I was always happy to be in lab even if I was not working. I would also like to thank Brian DeVree for all his help and endless conversations about GPCR theory and the implications of our research; a lot of those were documented here. I was extremely fortunate to have great collaborators that not only provided me with all the reagents I needed but also were key in making my project a success. -

Physiological and Pathophysiological Functions of the Ecto-Nucleotide Pyrophosphatase/Phosphodiesterase Family
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1638 (2003) 1–19 www.bba-direct.com Review Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family James W. Godinga, Bert Grobbenb, Herman Slegersb,* a Department of Pathology and Immunology, Monash Medical School, Monash University, Prahran 3181, Victoria, Australia b Department of Biomedical Sciences, Cellular Biochemistry, University of Antwerp, Universitaire Instelling Antwerpen, Universiteitplein 1, B-2610 Antwerpen-Wilrijk, Belgium Received 6 February 2003; received in revised form 20 March 2003; accepted 3 April 2003 Abstract The ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) multigene family contains five members. NPP1-3 are type II transmembrane metalloenzymes characterized by a similar modular structure composed of a short intracellular domain, a single transmembrane domain and an extracellular domain containing a conserved catalytic site. The short intracellular domain of NPP1 has a basolateral membrane-targeting signal while NPP3 is targeted to the apical surface of polarized cells. NPP4-5 detected by database searches have a predicted type I membrane orientation but have not yet been functionally characterized. E-NPPs have been detected in almost all tissues often confined to specific substructures or cell types. In some cell types, NPP1 expression is constitutive or can be induced by TGF-h and glucocorticoids, but the signal transduction pathways that control expression are poorly documented. NPP1-3 have a broad substrate specificity which may reflect their role in a host of physiological and biochemical processes including bone mineralization, calcification of ligaments and joint capsules, modulation of purinergic receptor signalling, nucleotide recycling, and cell motility. -

A Plant Proton-Pumping Inorganic Pyrophosphatase Functionally Complements the Vacuolar Atpase Transport Activity and Confers Bafilomycin Resistance in Yeast Jose R
A plant proton-pumping inorganic pyrophosphatase functionally complements the vacuolar ATPase transport activity and confers bafilomycin resistance in yeast Jose R. Perez-Castiñeira, Agustín Hernández, Rocio Drake, Aurelio Serrano To cite this version: Jose R. Perez-Castiñeira, Agustín Hernández, Rocio Drake, Aurelio Serrano. A plant proton-pumping inorganic pyrophosphatase functionally complements the vacuolar ATPase transport activity and con- fers bafilomycin resistance in yeast. Biochemical Journal, Portland Press, 2011, 437 (2), pp.269-278. 10.1042/BJ20110447. hal-00605257 HAL Id: hal-00605257 https://hal.archives-ouvertes.fr/hal-00605257 Submitted on 1 Jul 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Biochemical Journal Immediate Publication. Published on 26 May 2011 as manuscript BJ20110447 Title: A plant proton-pumping inorganic pyrophosphatase functionally complements the vacuolar ATPase transport activity and confers bafilomycin resistance in yeast Short title: Functional complementation of yeast vacuolar H+-ATPase by a H+-PPase Authors: José R. PÉREZ-CASTIÑEIRA, Agustín HERNÁNDEZ, Rocío DRAKE and Aurelio SERRANO1 Instituto de Bioquímica Vegetal y Fotosíntesis, Universidad de Sevilla-CSIC, Avda. Americo Vespucio, 49, 41092 Sevilla, Spain 1 To whom correspondence should be addressed (Phone: +-34-954489525. -

Supplementary Material
Supplementary material Figure S1. Cluster analysis of the proteome profile based on qualitative data in low and high sugar conditions. Figure S2. Expression pattern of proteins under high and low sugar cultivation of Granulicella sp. WH15 a) All proteins identified in at least two out of three replicates (excluding on/off proteins). b) Only proteins with significant change t-test p=0.01. 2fold change is indicated by a red line. Figure S3. TigrFam roles of the differentially expressed proteins, excluding proteins with unknown function. Figure S4. General overview of up (red) and downregulated (blue) metabolic pathways based on KEGG analysis of proteome. Table S1. growth of strain Granulicella sp. WH15 in culture media supplemented with different carbon sources. Carbon Source Growth Pectin - Glycogen - Glucosamine - Cellulose - D-glucose + D-galactose + D-mannose + D-xylose + L-arabinose + L-rhamnose + D-galacturonic acid - Cellobiose + D-lactose + Sucrose + +=positive growth; -=No growth. Table S2. Total number of transcripts reads per sample in low and high sugar conditions. Sample ID Total Number of Reads Low sugar (1) 15,731,147 Low sugar (2) 12,624,878 Low sugar (3) 11,080,985 High sugar (1) 11,138,128 High sugar (2) 9,322,795 High sugar (3) 10,071,593 Table S3. Differentially up and down regulated transcripts in high sugar treatment. ORF Annotation Log2FC GWH15_14040 hypothetical protein 3.71 GWH15_06005 hypothetical protein 3.12 GWH15_00285 tRNA-Asn(gtt) 2.74 GWH15_06010 hypothetical protein 2.70 GWH15_14055 hypothetical protein 2.66