Coronary Sinus Catheter Placement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prep for Practical II
Images for Practical II BSC 2086L "Endocrine" A A B C A. Hypothalamus B. Pineal Gland (Body) C. Pituitary Gland "Endocrine" 1.Thyroid 2.Adrenal Gland 3.Pancreas "The Pancreas" "The Adrenal Glands" "The Ovary" "The Testes" Erythrocyte Neutrophil Eosinophil Basophil Lymphocyte Monocyte Platelet Figure 29-3 Photomicrograph of a human blood smear stained with Wright’s stain (765). Eosinophil Lymphocyte Monocyte Platelets Neutrophils Erythrocytes "Blood Typing" "Heart Coronal" 1.Right Atrium 3 4 2.Superior Vena Cava 5 2 3.Aortic Arch 6 4.Pulmonary Trunk 1 5.Left Atrium 12 9 6.Bicuspid Valve 10 7.Interventricular Septum 11 8.Apex of The Heart 9. Chordae tendineae 10.Papillary Muscle 7 11.Tricuspid Valve 12. Fossa Ovalis "Heart Coronal Section" Coronal Section of the Heart to show valves 1. Bicuspid 2. Pulmonary Semilunar 3. Tricuspid 4. Aortic Semilunar 5. Left Ventricle 6. Right Ventricle "Heart Coronal" 1.Pulmonary trunk 2.Right Atrium 3.Tricuspid Valve 4.Pulmonary Semilunar Valve 5.Myocardium 6.Interventricular Septum 7.Trabeculae Carneae 8.Papillary Muscle 9.Chordae Tendineae 10.Bicuspid Valve "Heart Anterior" 1. Brachiocephalic Artery 2. Left Common Carotid Artery 3. Ligamentum Arteriosum 4. Left Coronary Artery 5. Circumflex Artery 6. Great Cardiac Vein 7. Myocardium 8. Apex of The Heart 9. Pericardium (Visceral) 10. Right Coronary Artery 11. Auricle of Right Atrium 12. Pulmonary Trunk 13. Superior Vena Cava 14. Aortic Arch 15. Brachiocephalic vein "Heart Posterolateral" 1. Left Brachiocephalic vein 2. Right Brachiocephalic vein 3. Brachiocephalic Artery 4. Left Common Carotid Artery 5. Left Subclavian Artery 6. Aortic Arch 7. -

Blood Flow DHO8 7.8, Pg
Blood Flow DHO8 7.8, pg. 190 HS1/2017-2018 Circuits •Pulmonary circuit –The blood pathway between the right of the heart, to the lungs, and back to the left side of the heart. •Systemic circuit –The pathway between the left side of the heart, to the body, and back to the right side of the heart. The Pathway of Blood •Superior & Inferior Vena •Left Atrium Cava •Mitral Valve •Right Atrium •Left Ventricle •Tricuspid Valve •Aortic Semilunar Valve •Right Ventricle •Aorta •Pulmonary Semilunar -Arteries Valve -Arterioles •Pulmonary Artery -Capillaries •Lungs -Venules –Pulmonary Arterioles -Veins –Pulmonary Capillaries –Pulmonary Venules •Pulmonary Vein Blood Flow Through Heart Do You Know? • When blood leaves the left atrium, where does it go next? a) Aorta b) Left ventricle c) Right atrium d) Pulmonary artery And the answer is….A Do You Know? • After blood leaves the right atrium, what valve prevents the back flow? a) Pulmonary b) Mitral c) Tricuspid d) Aortic And the answer is…C Do You Know? • The right ventricle is the chamber of the heart that pumps blood for the pulmonary circulation. Based on this information, blood from the right ventricle is on its way to the _____. a) Liver b) Lungs c) Hands and feet And the answer is…B Do You Know? • Which of the following is correct order of blood flow for the right side of the heart? a) RA, Tricuspid valve, RV, PSLV, pulmonary artery b) RA, PSLV, RV, Tricuspid valve, pulmonary artery c) RA, Tricuspid valve, RV, pulmonary artery , PSLV And the answer is…A Do You Know? • Which of the following is correct order of blood flow for the left side of the heart? a) LA, Bicuspid valve, LV, ASLV, aorta b) LA, ASLV, LV, Bicuspid valve, aorta c) LA, Bicuspid valve, LV, ASLV, aorta And the answer is…C. -

Severe Aortic Stenosis and the Valve Replacement Procedure
Severe Aortic Stenosis and the Valve Replacement Procedure A Guide for Patients and their Families If you’ve been diagnosed with severe aortic stenosis, you probably have a lot of questions and concerns. The information in this booklet will help you learn more about your heart, severe aortic stenosis, and treatment options. Your heart team will recommend which treatment option is best for you. Please talk with them about any questions you have. Table of Contents 4 About Your Heart 5 What Is Severe Aortic Stenosis? 5 What Causes Severe Aortic Stenosis? 7 What Are the Symptoms of Severe Aortic Stenosis? 8 Treatment Options for Severe Aortic Stenosis 10 Before a TAVR Procedure 12 What Are the Risks of TAVR? 2 3 About Your Heart What Is Severe See the difference between healthy and The heart is a muscle about the size of your fist. It is a pump that works nonstop to Aortic Stenosis? diseased valves send oxygen-rich blood throughout your entire body. The heart is made up of four The aortic valve is made up of two or three chambers and four valves. The contractions (heartbeats) of the four chambers push Healthy Valve the blood through the valves and out to your body. tissue flaps, called leaflets. Healthy valves open at every heart contraction, allowing blood to flow forward to the next chamber, and then close tightly to prevent blood from backing Pulmonic controls the flow of Aortic controls the flow of blood up. Blood flows in one direction only. This is Valve blood to the lungs Valve out of your heart to the important for a healthy heart. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Anomalous Middle Cardiac Vein Draining Into the Left Atrium
IMAGES IN THIS ISSUE Cardiovascular Disorders http://dx.doi.org/10.3346/jkms.2016.31.8.1179 • J Korean Med Sci 2016; 31: 1179-1180 Anomalous Middle Cardiac Vein Draining into the Left Atrium Jihyun Sohn,1 Young Soo Lee,2 Seung Pyo Hong,2 and Sung Hee Mun3 1Department of Cardiology, Kyungpook National University Hospital, Daegu, Korea; 2Department of Cardiology, Catholic University of Daegu, Daegu, Korea; 3Department of Radiology, Catholic University of Daegu, Daegu, Korea RV LV RA LA A B C LA CS RA PVLV LA MCV MCV LV D E Fig. 1. Cardiac computed tomography demonstrates the middle cardiac vein draining into the left atrium (yellow arrows). (A-C) Axial serial scan images of cardiac computed to- mography after contrast dye injection. (D) The 3-dimensional volume rendering image of cardiac computed tomography. (E) The curved planar reformation image of cardiac computed tomography. A 67-year-old woman was admitted for radiofrequency catheter oxygen supplement. Her electrocardiogram, chest X-ray, and ablation (RFCA) of paroxysmal atrial fibrillation. She had un- echocardiogram were within normal limits. She underwent derwent patch closure of atrial septal defect 7 years before. Her cardiac computed tomography for the left atrial reconstruction arterial blood oxygen saturation level was 94.6% without any before the RFCA. It revealed abnormal drainage of the middle © 2016 The Korean Academy of Medical Sciences. pISSN 1011-8934 This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -

Anatomy and Physiology of the Tricuspid Valve
JACC: CARDIOVASCULAR IMAGING VOL. 12, NO. 3, 2019 ª 2019 BY THE AMERICAN COLLEGE OF CARDIOLOGY FOUNDATION PUBLISHED BY ELSEVIER STATE-OF-THE-ART PAPER Anatomy and Physiology of the Tricuspid Valve a,b c c a,b Abdellaziz Dahou, MD, PHD, Dmitry Levin, BA, Mark Reisman, MD, Rebecca T. Hahn, MD SUMMARY An appreciation of the complex and variable anatomy of the tricuspid valve is essential to unraveling the pathophysiology of tricuspid regurgitation. A greater appreciation of normal and abnormal anatomy is important as new methods of treating the tricuspid regurgitation are developed. This review of tricuspid valve and right heart anatomy is followed by a discussion of the possible pathophysiology of secondary (functional) tricuspid regurgitation. (J Am Coll Cardiol Img 2019;12:458–68) © 2019 by the American College of Cardiology Foundation. ith the recognition of the impact of components: the leaflets, the papillary muscles, the W tricuspid regurgitation (TR) on outcomes chordal attachments, and the annulus (with attached in a number of disease states (1–5),inter- atrium and ventricle) (7,12–16).Theleaflets and their est in understanding this disease process has grown. relationship to the chordae and papillary muscle play To help understand the pathophysiology of TR and an important role in TV closure during systole but the role of interventions in treatment of the disease, also may be integrally related to RV size and function. an appreciation of the complex and variable anatomy TRICUSPID VALVE LEAFLETS. Although the TV is – ofthetricuspidvalve(TV)isessential(6 12).Thispa- typically composed of 3 leaflets of unequal size, in per reviews tricuspid and right heart anatomy, dis- many cases, 2 (bicuspid) or more than 3 leaflets may cusses the pathophysiology of secondary TR, be present as anatomic variants in healthy subjects summarizes the anatomic structures relevant to inter- (6,9) (Figure 2). -

Heart Valve Disease: Mitral and Tricuspid Valves
Heart Valve Disease: Mitral and Tricuspid Valves Heart anatomy The heart has two sides, separated by an inner wall called the septum. The right side of the heart pumps blood to the lungs to pick up oxygen. The left side of the heart receives the oxygen- rich blood from the lungs and pumps it to the body. The heart has four chambers and four valves that regulate blood flow. The upper chambers are called the left and right atria, and the lower chambers are called the left and right ventricles. The mitral valve is located on the left side of the heart, between the left atrium and the left ventricle. This valve has two leaflets that allow blood to flow from the lungs to the heart. The tricuspid valve is located on the right side of the heart, between the right atrium and the right ventricle. This valve has three leaflets and its function is to Cardiac Surgery-MATRIx Program -1- prevent blood from leaking back into the right atrium. What is heart valve disease? In heart valve disease, one or more of the valves in your heart does not open or close properly. Heart valve problems may include: • Regurgitation (also called insufficiency)- In this condition, the valve leaflets don't close properly, causing blood to leak backward in your heart. • Stenosis- In valve stenosis, your valve leaflets become thick or stiff, and do not open wide enough. This reduces blood flow through the valve. Blausen.com staff-Own work, CC BY 3.0 Mitral valve disease The most common problems affecting the mitral valve are the inability for the valve to completely open (stenosis) or close (regurgitation). -

Anatomy of the Heart
Anatomy of the Heart DR. SAEED VOHRA DR. SANAA AL-SHAARAWI OBJECTIVES • At the end of the lecture, the student should be able to : • Describe the shape of heart regarding : apex, base, sternocostal and diaphragmatic surfaces. • Describe the interior of heart chambers : right atrium, right ventricle, left atrium and left ventricle. • List the orifices of the heart : • Right atrioventricular (Tricuspid) orifice. • Pulmonary orifice. • Left atrioventricular (Mitral) orifice. • Aortic orifice. • Describe the innervation of the heart • Briefly describe the conduction system of the Heart The Heart • It lies in the middle mediastinum. • It is surrounded by a fibroserous sac called pericardium which is differentiated into an outer fibrous layer (Fibrous pericardium) & inner serous sac (Serous pericardium). • The Heart is somewhat pyramidal in shape, having: • Apex • Sterno-costal (anterior surface) • Base (posterior surface). • Diaphragmatic (inferior surface) • It consists of 4 chambers, 2 atria (right& left) & 2 ventricles (right& left) Apex of the heart • Directed downwards, forwards and to the left. • It is formed by the left ventricle. • Lies at the level of left 5th intercostal space 3.5 inch from midline. Note that the base of the heart is called the base because the heart is pyramid shaped; the base lies opposite the apex. The heart does not rest on its base; it rests on its diaphragmatic (inferior) surface Sterno-costal (anterior)surface • Divided by coronary (atrio- This surface is formed mainly ventricular) groove into : by the right atrium and the right . Atrial part, formed mainly by ventricle right atrium. Ventricular part , the right 2/3 is formed by right ventricle, while the left l1/3 is formed by left ventricle. -
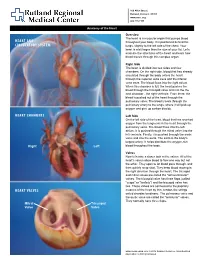
Heart and Circulatory System Heart Chambers
160 Allen Street Rutland, Vermont 05701 www.rrmc.org 802.775.7111 Anatomy of the Heart Overview The heart is a muscular organ that pumps blood HEART AND throughout your body. It is positioned behind the CIRCULATORY SYSTEM lungs, slightly to the left side of the chest. Your heart is a bit larger than the size of your fist. Let's examine the structures of the heart and learn how blood travels through this complex organ. Right Side The heart is divided into two sides and four chambers. On the right side, blood that has already circulated through the body enters the heart through the superior vena cava and the inferior vena cava. The blood flows into the right atrium. When this chamber is full, the heart pushes the blood through the tricuspid valve and into the the next chamber - the right ventricle. From there, the blood is pushed out of the heart through the pulmonary valve. The blood travels through the pulmonary artery to the lungs, where it will pick up oxygen and give up carbon dioxide. HEART CHAMBERS Left Side On the left side of the heart, blood that has received oxygen from the lungs enters the heart through the pulmonary veins. The blood flows into the left atrium. It is pushed through the mitral valve into the left ventricle. Finally, it is pushed through the aortic valve and into the aorta. The aorta is the body's largest artery. It helps distribute the oxygen-rich Right Left blood throughout the body. Valves Now let's take a closer look at the valves. -

Avalus™ Pericardial Aortic Surgical Valve System
FACT SHEET Avalus™ Pericardial Aortic Surgical Valve System Aortic stenosis is a common heart problem caused by a narrowing of the heart’s aortic valve due to excessive calcium deposited on the valve leaflets. When the valve narrows, it does not open or close properly, making the heart work harder to pump blood throughout the body. Eventually, this causes the heart to weaken and function poorly, which may lead to heart failure and increased risk for sudden cardiac death. Disease The standard treatment for patients with aortic valve disease is surgical aortic valve Overview: replacement (SAVR). During this procedure, a surgeon will make an incision in the sternum to open the chest and expose the heart. The diseased native valve is then Aortic removed and a new artificial valve is inserted. Once in place, the device is sewn into Stenosis the aorta and takes over the original valve’s function to enable oxygen-rich blood to flow efficiently out of the heart. For patients that are unable to undergo surgical aortic valve replacement, or prefer a minimally-invasive therapy option, an alternative procedure to treat severe aortic stenosis is called transcatheter aortic valve replacement (TAVR). The Avalus Pericardial Aortic Surgical Valve System is a next- generation aortic surgical valve from Medtronic, offering advanced design concepts and unique features for the millions of patients with severe aortic stenosis who are candidates for open- heart surgery. The Avalus Surgical Valve The Avalus valve, made of bovine tissue, is also the only stented surgical aortic valve on the market that is MRI-safe (without restrictions) enabling patients with severe aortic stenosis who have the Avalus valve to undergo screening procedures for potential co-morbidities.