In the Mediterranean Sea: an Update on Current Distribution with Two New Records from Sardinia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Bring Together and Discover Unesco About Us
BRING TOGETHER AND DISCOVER UNESCO ABOUT US Mirabilia Network links 17 Chambers of Commerce and as many UNESCO sites. Mirabilia Network is as a project which in 2017 became National Association. Mirabilia Network promotes lesser known destinations, “jewels” and territories bound by UNESCO recognition. Mirabilia Network wants to show different declinations of a territory, between history and culture, tradition and innovation, artistic craftsmanship and gastronomy. Mirabilia Network uses an “interconnected” language to enhance a new cultural tourism and to propose top itineraries without forgetting sustainability. Mirabilia Network develops a network between the Cities, also engaging the Municipal Administrations where our UNESCO sites are. NETWORK ROUTES CHAMBERS OF COMMERCE LINKED FOR THE PROMOTION OF CULTURAL TOURISM SITES IN ITALY MIRABILIA NETWORK BARI BENEVENTO CAMPOBASSO CASERTA CATANIA CROTONE Castel del Monte Complex of Saint Sofia Celebration of Mysteries Caserta Royale Palace Dome Square Ampollino, Sila National Park GENOVA GORIZIA IMPERIA ISERNIA LA SPEZIA MATERA Rolli of Genova Area of Collio Alps of the sea MAB Reserve Collemeluccio - Monterosso Al Mare - Cinque Terre Park of Rupestrian Churches Montedimezzo Alto Molise MESSINA PAVIA PERUGIA POTENZA RAGUSA SAVONA Salina Ponte Coperto Basilica of St. Francesco in Assisi Pollino National Park Val di Noto Beigua National Park SASSARI SIRACUSA TRIESTE UDINE VERONA Mount d’Accoddi Siracusa Dome Unity of Italy Square Patriarcal Basilica of Aquileia City 4 5 Must visit 1 Walk through the historical town of Bari and along the city walls. Your afternoon snack will be the typical focaccia baked in the bakeries located in the narrow alleys of the town. Visit the cathedral, the San Nicola church and the Svevo Castle. -

Bolbometopon Muricatum) in North Maluku Waters Muhammad J
DNA barcode and phylogenetics of green humphead parrotfish (Bolbometopon muricatum) in North Maluku waters Muhammad J. Achmad, Riyadi Subur, Supyan, Nebuchadnezzar Akbar Faculty of Fisheries and Marine Sciences, Khairun University, Ternate, North Maluku, Indonesia. Corresponding author: N. Akbar, [email protected] Abstract. The green humphead parrotfish (Bolbometopon muricatum) is one of the large species inhabiting coral reefs in North Maluku waters, Indonesia. The declining fish populations due to excessive fishing has caused the green humphead parrotfish to be listed in the Red List of IUCN in the vulnerable category since 2012. The species could be highly endangered, bordering extinction in the future. Studies on the genetic identification of green humphead parrotfish could be considered critical in the policy of sustainable conservation and fish culture. This research is designed for the identification and analysis of the genetic relationship of green humphead parrotfish based on the COI (cytochrome-c-oxidase subunit I) gene. DNA samples were collected from 4 locations in North Maluku, Ternate Island, Morotai Island, Bacan Island and Sanan Island. The DNA from samples was extracted and the COI gene was amplified using PCR (Polymerase Chain Reaction). Furthermore, the amplicon was sequenced to observe the similarities with the NCBI GenBank database. The results of this study showed that the green humphead parrotfish from this study had high similarities (98-100%) with the green humphead parrotfish with the reference access no. KY235362.1. Based on the phylogenetic tree, the green humphead parrotfish originating from North Maluku has a genetic relationship with the green humphead parrotfish from the database, but with different molecular characters. -

Reef Building Mediterranean Vermetid Gastropods: Disentangling the Dendropoma Petraeum Species Complex J
Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.1333 Zoobank: http://zoobank.org/25FF6F44-EC43-4386-A149-621BA494DBB2 Reef building Mediterranean vermetid gastropods: disentangling the Dendropoma petraeum species complex J. TEMPLADO1, A. RICHTER2 and M. CALVO1 1 Museo Nacional de Ciencias Naturales (CSIC), José Gutiérrez Abascal 2, 28006 Madrid, Spain 2 Oviedo University, Faculty of Biology, Dep. Biology of Organisms and Systems (Zoology), Catedrático Rodrigo Uría s/n, 33071 Oviedo, Spain Corresponding author: [email protected] Handling Editor: Marco Oliverio Received: 21 April 2014; Accepted: 3 July 2015; Published on line: 20 January 2016 Abstract A previous molecular study has revealed that the Mediterranean reef-building vermetid gastropod Dendropoma petraeum comprises a complex of at least four cryptic species with non-overlapping ranges. Once specific genetic differences were de- tected, ‘a posteriori’ searching for phenotypic characters has been undertaken to differentiate cryptic species and to formally describe and name them. The name D. petraeum (Monterosato, 1884) should be restricted to the species of this complex dis- tributed around the central Mediterranean (type locality in Sicily). In the present work this taxon is redescribed under the oldest valid name D. cristatum (Biondi, 1857), and a new species belonging to this complex is described, distributed in the western Mediterranean. These descriptions are based on a comparative study focusing on the protoconch, teleoconch, and external and internal anatomy. Morphologically, the two species can be only distinguished on the basis of non-easily visible anatomical features, and by differences in protoconch size and sculpture. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Sparisoma Rubripinne (Yellowtail Or Redfin Parrotfish)
UWI The Online Guide to the Animals of Trinidad and Tobago Ecology Sparisoma rubripinne (Yellowtail or Redfin Parrotfish) Family: Scaridae (Parrotfish) Order: Perciformes (Perch and Allied Fish) Class: Actinopterygii (Ray-finned Fish) Fig. 1. Yellowtail parrotfish, Sparisoma rubripinne. [http://www.tusentakk2.com/images/Other%20Photos/Underwater/Pec-Swimmers/YellowtailParrot.jpg, downloaded 11 April 2016] TRAITS. Parrotfish can be recognised by their beak-like fused teeth which are a tightly packed mosaic. The yellowtail or redfin parrotfish occurs in two colour phases. The terminal phase (Fig. 1) is the mature male with blue to green colour, a black spot at the base of the pectoral fin, and the tail fin with a yellow centre and translucent margin. The drab or initial phase includes either mature females or immature males, and has a light grey-brown colour with a white ventral region, alternate dark and pale bars along the chin, a yellow caudal peduncle (fin base) and tail fin, and red anal and pelvic fins (Fig. 2). The juvenile has a mottled pattern (Fig. 3). Fins include ten dorsal soft rays, nine dorsal spines, three anal spines and three anal soft rays. The length of the fish is 48cm in adult males (Randall, 1996). DISTRIBUTION. The species is found mostly in the western Atlantic and the Caribbean (Fig. 4). The fish is native to Trinidad and Tobago (Rocha et al., 2012). HABITAT AND ACTIVITY. Found in marine systems. The habitat ranges from reefs to sea grass beds of depth 1-15m. They inhabit shallow as well as rocky coral reefs, generally in the high energy areas of the reef. -

Marine Geophysical Research
Marine Geophysical Research Seabed mapping in the Pelagie Islands Marine Protected Area (Sicily Channel, southern Mediterranean) using Remote Sensing Object Based Image Analysis (RSOBIA) --Manuscript Draft-- Manuscript Number: MARI-D-18-00014R2 Full Title: Seabed mapping in the Pelagie Islands Marine Protected Area (Sicily Channel, southern Mediterranean) using Remote Sensing Object Based Image Analysis (RSOBIA) Article Type: Original Research Keywords: Multibeam bathymetry; backscatter; benthoscapes; seabed classification; ground- truth data; Posidonia oceanica; coralligenous habitat. Corresponding Author: Sara Innangi Istituto per l'ambiente marino costiero Consiglio Nazionale delle Ricerche ITALY Corresponding Author Secondary Information: Corresponding Author's Institution: Istituto per l'ambiente marino costiero Consiglio Nazionale delle Ricerche Corresponding Author's Secondary Institution: First Author: Sara Innangi First Author Secondary Information: Order of Authors: Sara Innangi Renato Tonielli Claudia Romagnoli Francesca Budillon Gabriella Di Martino Michele Innangi Roberta La Terza Tim Le Bas Claudio Lo Iacono Order of Authors Secondary Information: Funding Information: Abstract: AcceptedIn this paper we present the seabed maps of the shallow-water areas of Lampedusa and Linosa, belonging to the Pelagie Islands Marine Protected Area. Two surveys were carried out (“Lampedusa2015” and “Linosa2016”) to collect bathymetric and acoustic backscatter data through the use of a Reson SeaBat 7125 high-resolution multibeam system. Ground-truth -

1 Recreational Fishers Consistently Inform About Different
Recreational fishers consistently inform about different meridionalization dynamics of two Mediterranean subregions Valerio Sbragaglia1*, Jacopo Cerri1, Luca Bolognini2, Branko Dragičević3, Jakov Dulčić3, Fabio Grati2, 1,4 Ernesto Azzurro 1 Institute for Environmental Protection and Research (ISPRA), Via del Cedro 38, 57122 Livorno, Italy. 2 IRBIM, Institute of Biological Resources and Marine Biotechnologies - CNR, National Research Council, Ancona, Italy. 3 Institute of Oceanography and Fisheries, Split, Croatia. 4 Stazione Zoologica Anthon Dohrn, Napoles, Italy. * Corresponding author: [email protected] ABSTRACT Marine recreational fishers accumulate a vast amount of Local Ecological Knowledge (LEK) during their fishing activity that can be of paramount importance for monitoring how climate change affects the structure of biological communities. Here, we accessed the LEK of recreational anglers and recreational spearfishers to investigate the increase in the abundance of five northward expanding indigenous thermophilic fish species in two subregions of the Mediterranean Sea: the Adriatic/Ionian Seas and the Tyrrhenian/Ligurian Seas. We used an online survey administered through Twitter and Facebook between 2017 and 2018 to both Italian and Croatian recreational fishers. A total of 794 respondents completed the questionnaire (386 from the Adriatic/Ionian subregion and 408 from the Tyrrhenian/Ligurian one). Overall, the species perceived as most increasing in abundance were Pomatomus saltatrix (71% of replies) followed by Sphyraena -

Bhead Parrotfish Listing Petition
PETITION TO LIST THE Bumphead Parrotfish (Bolbometopon muricatum) UNDER THE U.S. ENDANGERED SPECIES ACT Photo: J.E. Maragos, USFWS Petition Submitted to the U.S. Secretary of Commerce, Acting Through the National Oceanic and Atmospheric Administration Fisheries Service & the U.S. Secretary of Interior, Acting through the U.S. Fish and Wildlife Service Petitioner: WildEarth Guardians 312 Montezuma Ave. Santa Fe, New Mexico 87501 (505) 988-9126 December 31, 2009 WildEarth Guardians Petition to List 1 the Bumphead Parrotfish Under the ESA Executive Summary The Bumphead Parrotfish (Bolbometopon muricatum) is a marine fish that feeds primarily on coral. It occurs in many countries in the Pacific and Indo-Pacific, including islands governed by the United States. While wide-ranging, scientists describe it as declining across its range and nearly eliminated from many areas. The primary threat has been overfishing, to which this fish is especially vulnerable due to its behavior of sleeping in large groups at night near reefs. Growing threats are coral bleaching and ocean acidification, both due to climate change. The Bumphead Parrotfish’s fate is tied to coral, as each fish consumes over 5 tons of coral every year. Coral consumed by the Parrotfish is excreted as coral sand, which is important to sustain the coral ecosystem, as well as providing beautiful white sand beaches enjoyed by tourists. Given the economic importance of tourism in the range of the Parrotfish, this species provides an invaluable ecosystem service to humans. An even more important way in which Parrotfish benefit humans is by protecting coral reef ecosystems, which are vital to safeguarding human coastal populations from impacts of extreme weather events. -

Of the FLORIDA STATE MUSEUM Biological Sciences
2% - p.*' + 0.:%: 4.' 1%* B -944 3 =5. M.: - . * 18 . .,:i -/- JL J-1.4:7 - of the FLORIDA STATE MUSEUM Biological Sciences Volume 24 1979 Number 1 THE ORIGIN AND SEASONALITY OF THE FISH FAUNA ON A NEW JETTY IN THE NORTHEASTERN GULF OF MEXICO ROBERT W. HASTINGS *S 0 4 - ' In/ g. .f, i»-ly -.Id UNIVERSITY OF FLORIDA - GAINESVILLE Numbers of the Bulletin of the Florida State Museum, Biological Sciences, are pub- lished at irregular intervals. Volumes contain about 300 pages and are not necessarily completed in any one calendar year. John William Hardy, Editor Rhoda J. Rybak, Managing Editor Consultants for this issue: Robert L. Shipp Donald P. deSylva Communications concerning purchase or exchange of the publications and all manuscripts should be addressed to: Managing Editor, Bulletin; Florida State Museum; University of Florida; Gainesville, Florida 32611. Copyright © 1979 by the Florida State Museum of the University of Florida. This public document was promulgated at an annual cost of $3,589.40, or $3.589 per copy. It makes available to libraries, scholars, and all interested persons the results of researches in the natural sciences, emphasizing the circum-Caribbean region. Publication date: November 12, 1979 Price, $3.60 THE ORIGIN AND SEASONALITY OF THE FISH FAUNA ON A NEW JETTY IN THE NORTHEASTERN GULF OF MEXICO ROBERT W. HASTINGS1 SYNOPSIS: The establishment of the fish fauna on a new jetty at East Pass at the mouth of Choctawhatchee Bay, Okaloosa County, Florida, was studied from June, 1968, to January, 1971. Important components of the jetty fauna during its initial stages of development were: (a) original residents that exhibit some attraction to reef habitats, including some sand-beach inhabitants, several pelagic species, and a few ubiquitous estuarine species; and (b) reef fishes originating from permanent populations on offshore reefs. -

Marine Turtles
UNEP/MED IG.24/22 Page 372 Decision IG.24/7 Strategies and Action Plans under the Protocol concerning Specially Protected Areas and Biological Diversity in the Mediterranean, including the SAP BIO, the Strategy on Monk Seal, and the Action Plans concerning Marine Turtles, Cartilaginous Fishes and Marine Vegetation; Classification of Benthic Marine Habitat Types for the Mediterranean Region, and Reference List of Marine and Coastal Habitat Types in the Mediterranean The Contracting Parties to the Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and its Protocols at their 21st Meeting, Recalling the outcome document of the United Nations Conference on Sustainable Development, entitled “The future we want”, endorsed by the General Assembly in its resolution 66/288 of 27 July 2012, in particular those paragraphs relevant to biodiversity, Recalling also General Assembly resolution 70/1 of 25 September 2015, entitled “Transforming our world: the 2030 Agenda for Sustainable Development”, and acknowledging the importance of conservation, the sustainable use and management of biodiversity in achieving the Sustainable Development Goals, Recalling further the United Nations Environment Assembly resolutions UNEP/EA.4/Res.10 of 15 March 2019, entitled “Innovation on biodiversity and land degradation”, Bearing in mind the international community’s commitment expressed in the Ministerial Declaration of the United Nations Environment Assembly at its fourth session to implement sustainable ecosystems -
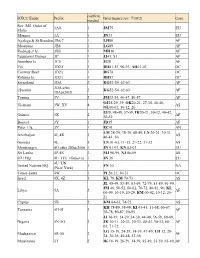
DXCC Entity Prefix Confirm. Needed Grid Square.Rev: 7/30/21 Cont. Sov
confirm. DXCC Entity Prefix Grid Square.rev: 7/30/21 Cont. needed Sov. Mil. Order of 1A0 1 JM75 EU Malta Monaco 3A 1 JN33 EU Agalega & St.Brandon 3B6,7 1 LH89 AF Mauritius 3B8 1 LG89 AF Rodriguez Is. 3B9 1 MH10 AF Equatorial Guinea 3C 1 JJ41, 51 AF Annobon Is. 3C0 1 JI28 AF Fiji 3D2/f 3 RH81-83, 90-93, AH01-03 OC Conway Reef 3D2/c 1 RG78 OC Rotuma Is. 3D2/r 1 RH87 OC Swaziland 3DA 2 KG52-54, 62-63 AF 3DA after eSwatini 2 KG52-54, 62-63 AF 18Apr2018 Tunisia 3V 2 JM33-34, 40-47, 50-57 AF OJ28-29, 39, OK20-21, 27-38, 40-46, Vietnam 3W, XV 4 AS OL00-01, 10-12, 20 IJ39, 48-49, 57-59, IK20-21, 30-32, 40-42, Guinea 3X 2 AF 50-52 Bouvet 3Y 1 JD15 AF Peter 1 Is. 3Y 1 EC41 AN LM 28-29, 38-39, 48-49, LN 20-21, 30-31. Azerbaijan 4J, 4K 3 AS 40-41, 50 Georgia 4L 3 LN 01-03, 11-13, 21-22, 31-32 AS Montenegro 4O after 28Jun2006 1 JN 91-93, KN 02-03 EU Sri Lanka 4P-4S 2 MJ 96-99, NJ 06-09 AS ITU HQ 4U_ITU (Geneva) 1 JN 36 EU 4U_UN United Nations HQ 1 FN 30 NA (New York) Timor-Leste 4W 1 PI 20-21, 30-31 OC Israel 4X, 4Z 3 KL 79, KM 70-73 AS JL 45-49, 53-59, 63-69, 72-79, 83-89, 91-99, JM 40, 50-52, 60-62, 70-72, 80-81, 90, KL Libya 5A 2 AF 00-09, 10-19, 20-29, KM 00-02, 10-12, 20- 21 Cyprus 5B 2 KM 64-65, 74-75 AS KH 78-89, 98-99, KI 43-45, 51-58, 60-67, Tanzania 5H-5I 3 AF 70-78, 80-87, 90-95 JJ 16-19, 24-29, 34-39, 44-49, 56-59, 68-69, Nigeria 5N-5O 3 JK 10-11, 20-23, 30-33, 40-43, 50-53, 60- AF 63, 71-72 LG 15-19, 24-29, 34-39, 47-49, LH 12, 20- Madagascar 5R-5S 2 AF 24, 30-36, 40-48, 53-56 Mauritania 5T 2 IK 16-19, 26-29, 34-39, 45-49, 55-59, 65-69, -

Life on the Coral Reef
Coral Reef Teacher’s Guide Life on the Coral Reef Life on the Coral Reef THE CORAL REEF ECOSYSTEM The muddy silt drifts out to sea, covering the nearby Coral reefs provide the basis for the most productive coral reefs. Some corals can remove the silt, but many shallow water ecosystem in the world. An ecosystem cannot. If the silt is not washed off within a short pe- is a group of living things, such as coral, algae and riod of time by the current, the polyps suffocate and fishes, along with their non-living environment, such die. Not only the rainforest is destroyed, but also the as rocks, water, and sand. Each influences the other, neighboring coral reef. and both are necessary for the successful maintenance of life. If one is thrown out of balance by either natural Reef Zones or human-made causes, then the survival of the other Coral reefs are not uniform, but are shaped by the is seriously threatened. forces of the sea and the structure of the sea floor into DID YOU KNOW? All of the Earth’s ecosystems are a series of different parts or reef zones. Understand- interrelated, forming a shell of life that covers the ing these zones is useful in understanding the ecol- entire planet – the biosphere. For instance, if too many ogy of coral reefs. Keep in mind that these zones can trees are cut down in the rainforest, soil from the for- blend gradually into one another, and that sometimes est is washed by rain into rivers that run to the ocean.