Evaluation of the Conservation Status of the Croatian Posavina Horse Breed Based on Pedigree and Microsatellite Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reference Values and Influence of Sex and Age on Hemogram and Clinical Biochemistry in Protected and Endangered Murinsulaner Horses
VETER.INARSKI ARHIV 89 (1), 25-42, 2019 DOI: 10.24099/vet.arhiv.0479 Reference values and influence of sex and age on hemogram and clinical biochemistry in protected and endangered Murinsulaner horses Nikica Prvanović Babić1*, Antun Kostelić2, Branimir Novak3, Dragica Šalamon2, Bruna Tariba2, Nino Maćešić1, Tugomir Karadjole1, Goran Bačić1, and Ljiljana Bedrica4 1Clinic for Obstetrics and Reproduction, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia 2Department for Animal Science, Faculty of Agriculture, University of Zagreb, Zagreb, Croatia 3Veterinary station ʺBioinstitutʺ, Čakovec, Croatia 4Clinic for Internal Deseases, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia ________________________________________________________________________________________ PRvANović BABić, N., A. KosTeLić, B. NovAK, D. ŠALAMoN, B. TARiBA, N. MAćeŠić, T. KARADjoLe, G. BAčić, Lj. BeDRicA: Reference values and influence of sex and age on hemogram and clinical biochemistry in protected and endangered Murinsulaner horses. vet. arhiv 89, 25-42, 2019. ABsTRAcT The aim of this study was to establish the exact haematological and clinical biochemistry reference values for horses of the Murinsulaner breed, using the complete population in Croatia (n = 33) for the sampling. Haemoglobin concentrations (HB), red blood cell count (RBC), white blood cell count (WBC), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular haemoglobin concentration (MCHC), mean corpuscular haemoglobin (MCH), platelet count and differential white blood cell counts were determined. Clinical biochemistry included the activity of GGT, AST, ALT, CK, ALP, LDH and AMYL, concentrations of glucose (GLUC), total protein (TP), albumin (ALB), cholesterol (CHOL), triglycerides (TRI), calcium (Ca), phosphorus (P), magnesium (Mg), iron (Fe), urea (UREA), creatinine (CREA) and total bilirubin (TBIL). The animals were sorted into groups according to sex and age, and analysis of variance (ANOVA) was preformed using the GLM procedure and Tukey’s studentized range test. -

Kopitari – Godišnje Izvješće 2019
Hrvatska agencija za poljoprivredu i hranu Croatian Agency for Agriculture and Food Centar za stočarstvo Centre for Livestock Breeding KOPITARI EQUIDAE BREEDING GODIŠNJE IZVJEŠĆE ZA 2019. GODINU ANNUAL REPORT FOR 2019. Hrvatska agencija za poljoprivredu i hranu, Centar za stočarstvo Pravna osnova / Legal basis Zakon o Hrvatskoj agenciji za poljoprivredu i hranu (NN 111/2018) Law on the Croatian Agency for Agriculture and Food Izdavač / Publisher Hrvatska agencija za poljoprivredu i hranu Croatian Agency for Agriculture and Food Adresa / Address Vinkovačka cesta 63 c, 31 000 Osijek Telefon / Phone +385 (0)31 275 200 E-mail [email protected] Web www.hapih.hr Odgovorna osoba izdavača doc. dr. sc. Krunoslav Dugalić Responsible person of the publisher Uredništvo / Editorial Centar za stočarstvo Centre for Livestock Breeding Adresa / Address Vinkovačka cesta 63 c, 31 000 Osijek Telefon / Phone +385 (0)31 815 019 E-mail [email protected] Glavni i odgovorni urednik / Chief editor doc. dr. sc. Krunoslav Dugalić Urednici / Editors Davor Pašalić, dr.med.vet. dr. sc. Zdenko Ivkić dr. sc. Drago Solić Prikupljanje podataka Područni uredi Centra za stočarstvo Data collected by Regional offices of Centre for Livestock Breeding Obrada podataka Ministarstvo poljoprivrede Data processing Uprava za stočarstvo i kvalitetu hrane Ministry of Agriculture Directorate for Livestock and Food Quality Oblikovanje / Design Glas Slavonije d.d., Osijek Tisak / Printing Glas Slavonije d.d., Osijek ISSN 2670-8787 Naklada / Edition 300 Molimo korisnike da Users are kindly requested -

List of Horse Breeds 1 List of Horse Breeds
List of horse breeds 1 List of horse breeds This page is a list of horse and pony breeds, and also includes terms used to describe types of horse that are not breeds but are commonly mistaken for breeds. While there is no scientifically accepted definition of the term "breed,"[1] a breed is defined generally as having distinct true-breeding characteristics over a number of generations; its members may be called "purebred". In most cases, bloodlines of horse breeds are recorded with a breed registry. However, in horses, the concept is somewhat flexible, as open stud books are created for developing horse breeds that are not yet fully true-breeding. Registries also are considered the authority as to whether a given breed is listed as Light or saddle horse breeds a "horse" or a "pony". There are also a number of "color breed", sport horse, and gaited horse registries for horses with various phenotypes or other traits, which admit any animal fitting a given set of physical characteristics, even if there is little or no evidence of the trait being a true-breeding characteristic. Other recording entities or specialty organizations may recognize horses from multiple breeds, thus, for the purposes of this article, such animals are classified as a "type" rather than a "breed". The breeds and types listed here are those that already have a Wikipedia article. For a more extensive list, see the List of all horse breeds in DAD-IS. Heavy or draft horse breeds For additional information, see horse breed, horse breeding and the individual articles listed below. -

Biodiversity of Arabian Horses in Syria
Biodiversity of Arabian horses in Syria Dissertation zur Erlangung des akademischen Grades Doctor rerum agriculturarum (Dr. rer. agr.) eingereicht an der Lebenswissenschaftlichen Fakultät der Humboldt Universität zu Berlin von M.Sc. Saria Almarzook Präsidentin der Humboldt-Universität zu Berlin Prof. Dr. Sabine Kunst Dekan der Humboldt-Universität zu Berlin Prof. Dr. Bernhard Grimm Gutachterin/Gutachter Prof. Dr. Gudrun Brockmann Prof. Dr. Dirk Hinrichs Prof. Dr. Armin Schmitt Tag der mündlichen Prüfung: 17. September 2018 Dedication This research is dedicated to my homeland …Syria Contents Zusammenfassung ................................................................................................................... I Summary ............................................................................................................................... VI List of publications and presentations .................................................................................. XII List of abbreviations ............................................................................................................ XIII List of figures ....................................................................................................................... XIV List of tables ......................................................................................................................... XV 1. General introduction and literature review ..................................................................... 1 1.1. Domestication and classification -

History-Of-Breeding-And-Training-Of-The-Kladruber-Horses
History of Breeding and Training of the Kladruber Horses The Kladruber horse is the only breed of the original ceremonial horses still bred that is the only draught horse breed in the world originated, bred and trained for drawing carriages of the social elites. Thanks to the Habsburg conservatism and unchanged breeding goal, the Kladruber horse has preserved its original “baroque” appearance from the 18th century to date. It still bears the traits of the original, but now extinct breeds (old Spanish horse and old Italian horse) which were at its beginning and from medieval times until the 18th century influenced the stock in most European countries and colonies and by the end of the 18th century were extinct. Even though there are only limited opportunities for ceremonial carriage horses to be used at (now the most frequent breeds are warmblooded horses for sport) the Kladruber horse breed has been preserved and still serves its original purpose for example at the Danish Royal Court and it is also used for state functions. Horse breeds are divided into primitive (indigenous) and intentionally designed (on the basis of targeted selective breeding) however some breeds oscillate between these two main types. Then the horse breeds are divided according to their purpose such as draught horses which the carriage horses fall into (weight up to 1200 kg), riding horses (up to 800kg) and pack horses (less than 500 kg). A new horse breed came into existence either in a particular area, using the same genetic material and the effect of the external conditions and climate (most of the breeds started in this way) or it came into existence in a single place – at a dedicated stud farm with a clearly defined breeding goal using particular horses of selected breeds imported for this sole purpose and applying the knowledge of selective breeding available at that time as well as the knowledge of local natural conditions and climate. -

D a G R Dan a N Ge Res Nub Nim Ne Sour Bian M a Tic Rce N L C Es
Proceedings of 28th Annual Meeting of DAGENE Danubian AAnn i m a l G e netic Resourrces Volume 2 (2017) DAGENE International Association for the Conservation of Animal Breeds in the Danube Region 1078 Budapest, István street 2. Hungary Proceedings of 28th Annual Meeting of DAGENE Danubian A n i m a l G e n e tic Resources Volume 2 (2017) DAGENE International Association for the Conservation of Animal Breeds in the Danube Region 1078 Budappest, István street 2. Hungary 2 Danubian Animal Genetic Resources Volume 2 (2017) “Tradition and innovation in preservation of autochthonous breeds” Proceedings of 28th Annual Meeting of DAGENE in Pazin, Croatia from 26th to 29th of April 2017 DAGENE International Association for the Conservation of Animal Breeds in the Danube Region 1078 Budapest, István street 2. Hungary 3 Danubian Animal Genetic Resources Volume 2 (2017) Person responsible for edition: Editor-in-Chief: András GÁSPÁRDY (Hungary) Editorial board: President: Ante IVANKOVIČ (Croatia) Pál HAJAS (Hungary) Beate BERGER (Austria) Mojca SIMČIČ (Slovenia) Marcel MATIUTI (Romania) Zsolt BECSKEI (Serbia) Iudith IPATE (Romania) János POSTA (Hungary) Technical editor: Kata ANNUS Editorial office: DAGENE - International Association for the Conservation of Animal Breeds in the Danube Region, 1078 Budapest, István u. 2. [email protected] - www.dagene.eu Publisher: DAGENE - International Association for the Conservation of Animal Breeds in the Danube Region, 1078 Budapest, István u. 2. [email protected] - www.dagene.eu Person responsible for publishing: Pál HAJAS president of DAGENE Printed by A/3 Printing and Publishing Ltd., Péter MOHAI HU ISSN: 2498-5910 This journal, founded 2016 is the official leaflet of the Annual Scientific Conference of DAGENE, as well as the publication medium for the yearly activity of DAGENE members. -

Special Issue Horse Genetics DIRECTOR’S Message
CENTER FOR EQUINE HEALTH SCHOOL OF VETERINARY MEDICINE • UNIVERSITY OF CALIFORNIA, DAVIS SUMMER 2020 Special Issue Horse Genetics DIRECTOR’S Message s an equine genetics researcher, I am particularly excited to share A this special issue of the Horse Report with you. Inside, you will find a roadmap to many of the currently available equine genetic tests, including the AQHA “five-panel” test, and more. The equine genome sequence was published in 2009, the result of a years- long collaborative effort by the international equine research community. This resource drastically changed how researchers approach equine genetics and accelerated the rate of discovery. Increased availability and affordability allowed the application of advanced molecular tools to equine diseases and traits. As a result, genetic tests are available in a variety of breeds. Most available tests are for simple, Mendelian diseases and traits – those caused by a single gene or locus. Complex diseases and traits likely involve more than one gene and may be influenced by environmental effects. The 2018 release of a new equine genome sequence assembly, coupled with cost reductions that make whole-genome sequencing possible for large numbers of horses, are enabling research in these areas. As an equine geneticist and veterinarian, I am especially interested in applying whole genome sequencing and advanced diagnostic tools to equine precision medicine. This highly individualized approach will focus on early detection and prevention of disease, taking into account both genetic information and environmental factors. The idea is to target individuals based on their clinical condition as well as their unique body chemistry and genetics. -

A View of the Horse from the Classical Perspective the Penn Museum Collection by Donald White
A View of the Horse from the Classical Perspective The Penn Museum Collection by donald white quus caballus is handsomely stabled in tive how-to manual On Horsemanship (“The tail and mane the University of Pennsylvania Museum of should be washed, to keep the hairs growing, as the tail is used Archaeology and Anthropology. From the to swat insects and the mane may be grabbed by the rider Chinese Rotunda’s masterpiece reliefs portray- more easily if long.”) all the way down to the 9th century AD ing two horses of the Chinese emperor Taizong Corpus of Greek Horse Veterinarians, which itemizes drugs for Eto Edward S. Curtis’s iconic American Indian photographs curing equine ailments as well as listing vets by name, Greek housed in the Museum’s Archives, horses stand with man and Roman literature is filled with equine references. One in nearly every culture and time-frame represented in the recalls the cynical utterance of the 5th century BC lyric poet Museum’s Collection (pre-Columbian America and the Xenophanes from the Asia Minor city of Colophon: “But if northern polar region being perhaps the two most obvious cattle and horses and lions had hands, or were able to do the exceptions). Examples drawn from the more than 30,000 work that men can, horses would draw the forms of the gods like Greek, Roman, and Etruscan vases, sculptures, and other horses” (emphasis added by author). objects in the Museum’s Mediterranean Section serve here as The partnership between horse and master in antiquity rested a lens through which to view some of the notable roles the on many factors; perhaps the most important was that the horse horse played in the classical Mediterranean world. -

Breeds in Republic of Croatia
Legislative and institutional framework for the protection of native (autochthonous) breeds in Republic of Croatia Jasna Jeremić1, State Institute for Nature Protection; Mirna Dadić1, Ministry of Agriculture, Fishery and Rural Development; Ante Ivanković ², Agronomy Faculty University of Zagreb; Dražen Cerjanec³, Ministry of Agriculture, Fishery and Rural Development ¹State Institute for Nature Protection, Trg Mažuranića 5, 10 000 Zagreb, Croatia Phone: +385 1 5502 921 E-mail address: [email protected] ¹Ministry of Agriculture, Fishery and Rural Development, Ul. Grada Vukovara, 10 000 Zagreb, Croatia Phone: +385 1 6106 693 E-mail address: [email protected] ²Agronomy Faculty University of Zagreb, Svetošimunska cesta 25, 10 000 Zagreb, Croatia Phone:+385 1 2393 991 E-mail address: [email protected] ³Ministry of Agriculture, Fishery and Rural Development, Ul. Grada Vukovara, 10 000 Zagreb, Croatia Phone: +385 1 6106 971 E-mail address: [email protected] Key words: institutional framework, legislative framework, native breeds, protection, subsidies The protection of Croatian native breeds and varieties is covered by many Institutions and Acts. The Ministry of Agriculture, Fishery and Rural development addresses the issue through several responsible units: Department for Veterinary Science; Department of Agriculture; Department for Agricultural Policy, EU and International Relations; Department for Sustainable Rural Development and Direction for SAPARD/IPARD programme, Department for Market and Structural Support in Agriculture, Fishery and Rural Development and Department for Inspectional Affairs. The Ministry of Environmental Protection, Physical Planning and Construction governs the same issues through the Directorate for Atmosphere and Waste Management, Directorate for Environmental Assessment and Industrial Pollution, Directorate for the European Union, Directorate for International Cooperation and Sustainable Development, Directorate for Physical Planning, Inspectional Affairs etc. -
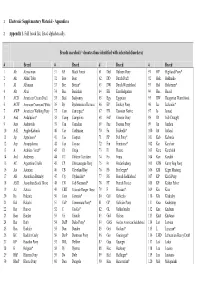
Electronic Supplementary Material - Appendices
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

Multiple Pregnancy in Mares
MENDELNET 2016 MULTIPLE PREGNANCY IN MARES MARIE IMRICHOVA Department of Animal Breeding Mendel University in Brno Zemedelska 1, 613 00 Brno CZECH REPUBLIC [email protected] Abstract: Mare is in terms of reproduction described as uniparous, it means that she has one foal. More embryos, fetuses or foals represents non – physiological phenomenon and as such it brings a lot of complications. In terms of the etiology is discussed as a predisposing factor mare’s breed, the most common is a higher incidence associated with Thoroughbreds. Although in context of multiple pregnancies often mentioned is mare‘s age. The aim of this study was to evaluate the incidence and results of multiple pregnancy in Thoroughbred and Old Kladruber Horse mares. It was found 323 records of multiple pregnancies in Thoroughbred and 48 in Old Kladruber Horse mares and the result of the multiple pregnancy was in Thoroughbreds in 258 cases twin abortion, in 45 cases parturition of 2 dead foals, 15 records of parturition 1 live and 1 dead foal and in 5 cases it was the parturition of 2 live foals. In Old Kladruber Horse twin abortion was recorded 36 times, in 7 cases 2 dead foals and in 5 cases 2 live foals. Key Words: horse, breeding, reproduction, pregnancy, twins INTRODUCTION The reproduction standard in horse breeding is the fundamental informative factor affecting its success and profitability. One of the factors that can decide about the reproduction outcome is also multiple pregnancy. Multiple pregnancy basically may occur naturally either by spontaneous division of embryos, or by fertilization of two oocytes after multiple ovulation. -

Lipizzan Laurels United States Lipizzan Federation©
Lipizzan Laurels United States Lipizzan Federation© Awards Program The United States Lipizzan Federation (USLF) recognizes Lipizzans/XL Lipizzans competing in events against all breeds with two special award programs—the USLF Lipizzan/XL Lipizzan Laurels Award and the USLF Lipizzan/XL Lipizzan Star Award. Award participants automatically are entered in both programs when they submit results. The USLF Lipizzan Laurels presents a Lipizzan Laurels Award for outstanding performance by a Lipizzan/XL Lipizzan horse in several sections within the program’s ten major disciplines. Junior Exhibitor Awards are also presented in all ten disciplines. Lipizzan Laurels awards are tabulated on an annual basis during the competition year, which runs from November 1st of the previous year to October 31st of the current year. The Lipizzan/XL Lipizzan Star Award is a lifetime award presented to each Lipizzan/XL Lipizzan meeting Star requirements. Lipizzan/XL Lipizzan horses may take as many years as needed to earn Star points. The owner of the achieving horse will receive an official bronze, silver, gold, or platinum Star when they have accumulated the necessary points in their discipline. Stars are presented in ten disciplines: Show, Competitive Trail, Endurance, Dressage, Eventing, Working Western, Carriage Pleasure, Combined Driving, Western Dressage, and Working Equitation. Horses who earn five of the ten bronze Stars are presented with the USLF Lipizzan/XL Lipizzan Sport Horse Award. 1 GENERAL GUIDELINES To be eligible for these awards, horses must be registered with the USLF at the time scores are earned. Both the owner and all riders or drivers of the horse must be current USLF members at the time scores are earned.