Mitogenome Analysis of Four Lamiinae Species (Coleoptera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LONGHORN BEETLE CHECKLIST - Beds, Cambs and Northants
LONGHORN BEETLE CHECKLIST - Beds, Cambs and Northants BCN status Conservation Designation/ current status Length mm In key? Species English name UK status Habitats/notes Acanthocinus aedilis Timberman Beetle o Nb 12-20 conifers, esp pine n ox-eye daisy and other coarse herbaceous plants [very recent Agapanthia cardui vr 6-14 n arrival in UK] Agapanthia villosoviridescens Golden-bloomed Grey LHB o f 10-22 mainly thistles & hogweed y Alosterna tabacicolor Tobacco-coloured LHB a f 6-8 misc deciduous, esp. oak, hazel y Anaglyptus mysticus Rufous-shouldered LHB o f Nb 6-14 misc trees and shrubs y Anastrangalia (Anoplodera) sanguinolenta r RDB3 9-12 Scots pine stumps n Anoplodera sexguttata Six-spotted LHB r vr RDB3 12-15 old oak and beech? n Anoplophora glabripennis Asian LHB vr introd 20-40 Potential invasive species n Arhopalus ferus (tristis) r r introd 13-25 pines n Arhopalus rusticus Dusky LHB o o introd 10-30 conifers y Aromia moschata Musk Beetle o f Nb 13-34 willows y Asemum striatum Pine-stump Borer o r introd 8-23 dead, fairly fresh pine stumps y Callidium violaceum Violet LHB r r introd 8-16 misc trees n Cerambyx cerdo ext ext introd 23-53 oak n Cerambyx scopolii ext introd 8-20 misc deciduous n Clytus arietus Wasp Beetle a a 6-15 misc, esp dead branches, posts y Dinoptera collaris r RDB1 7-9 rotten wood with other longhorns n Glaphyra (Molorchus) umbellatarum Pear Shortwing Beetle r o Na 5-8 misc trees & shrubs, esp rose stems y Gracilia minuta o r RDB2 2.5-7 woodland & scrub n Grammoptera abdominalis Black Grammoptera r r Na 6-9 -

31 First Record of Batocera Rufomaculata (De Geer, 1775) from Sunderban Biosphere Reserve, West Bengal
International Journal of Entomology Research ISSN: 2455-4758 www.entomologyjournals.com Volume 1; Issue 3; March 2016; Page No. 31-32 First record of Batocera rufomaculata (De Geer, 1775) from Sunderban biosphere reserve, West Bengal 1 Bulganin Mitra, 2 Udipta Chakraborti, 3 Olive Biswas, 4 Sankarsan Roy, 5 Kaushik Mallick, 6 Priyanka Das 1, 2, 3, 4, 6 Zoological Survey of India, Prani Vigyan Bhawan, M-Block, New Alipore, Kolkata. 5 Post Graduate Department of Zoology, Asutosh College, Kolkata Abstract Studies on Longhorn beetles (Coleoptera) in Sunderban region is very poor. Altogether, 8 species under 3 subfamilies are already reported from Sunderban Biosphere Reserve. Present communication reports Batocera rufomaculata (De Geer, 1775) for the first time from this Biosphere reserve. Keywords: Sunderban Biosphere Reserve, Cerambycidae, Lamiinae, Batocera Introduction Sunderban region in India is 9600 sq km (4200 sq km of Reserved Forest and 5400 sq km of non-forest, inhabited region) which constitutes the Sunderban Biosphere Reserve (SBR). Indian Sunderban is bound on the west by river Muriganga and on the east by rivers Harinbhahga and Raimangal. Administrative boundary of the Sunderban is spread over two districts i.e. North 24-Parganas (Hingalganj, Hasnabad, Haroa, Sandeskhali - I,II, and Minakhan blocks) and South 24-Parganas (Sagar, Namkhana, Kakdwip, Patharpratima, Kultali, Mathurapur-I,II, Jaynagar-I,II, Canning-I,II, Basanti and Gosaba blocks).The extent of mangrove Reserve Forests in Indian Sunderban is around 4260 sq km, out of which 55% is under land vegetation cover and balance 45% is under water body/ inter-tidal zone. Studies on beetles and weevils (Coleoptera) in Sunderban region is very poor. -

Transcriptome and Gene Expression Analysis of Rhynchophorus Ferrugineus (Coleoptera: Curculionidae) During Developmental Stages
Transcriptome and gene expression analysis of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) during developmental stages Hongjun Yang1,2, Danping Xu1, Zhihang Zhuo1,2,3, Jiameng Hu2 and Baoqian Lu4 1 College of Life Science, China West Normal University, Nanchong, Sichuan, China 2 Key Laboratory of Genetics and Germplasm Innovation of Tropical Special Forest Trees and Ornamental Plants, Ministry of Education, Key Laboratory of Germplasm Resources Biology of Tropical Special Ornamental Plants of Hainan Province, College of Forestry, Hainan University, Haikou, Hainan, China 3 Key Laboratory of Integrated Pest Management on Crops in South China, Ministry of Agriculture, South China Agricultural University, Guangzhou, Guangdong, China 4 Key Laboratory of Integrated Pest Management on Tropical Crops, Ministry of Agriculture China, Environ- ment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Sciences, Haikou, Hainan, China ABSTRACT Background. Red palm weevil, Rhynchophorus ferrugineus Olivier, is one of the most destructive pests harming palm trees. However, genomic resources for R. ferrugineus are still lacking, limiting the ability to discover molecular and genetic means of pest control. Methods. In this study, PacBio Iso-Seq and Illumina RNA-seq were used to generate transcriptome from three developmental stages of R. ferrugineus (pupa, 7th-instar larva, adult) to increase the understanding of the life cycle and molecular characteristics of the pest. Results. Sequencing generated 625,983,256 clean reads, from which 63,801 full-length transcripts were assembled with N50 of 3,547 bp. Expression analyses revealed 8,583 differentially expressed genes (DEGs). Moreover, gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis revealed that these Submitted 5 March 2020 Accepted 29 September 2020 DEGs were mainly related to the peroxisome pathway which associated with metabolic Published 2 November 2020 pathways, material transportation and organ tissue formation. -

Exploring Flat Faced Longhorn Beetles (Cerambycidae: Lamiinae) from the Reserve Forests of Dooars, West Bengal, India
Hindawi Publishing Corporation ISRN Entomology Volume 2013, Article ID 737193, 8 pages http://dx.doi.org/10.1155/2013/737193 Research Article Exploring Flat Faced Longhorn Beetles (Cerambycidae: Lamiinae) from the Reserve Forests of Dooars, West Bengal, India Sumana Saha,1 Hüseyin Özdikmen,2 Manish Kanti Biswas,3 and Dinendra Raychaudhuri4 1 Department of Zoology, Darjeeling Government College, Government of West Bengal, Darjeeling, West Bengal 734101, India 2 Gazi Universitesi,¨ Fen-Edebiyat Fakultesi,¨ Biyoloji Bol¨ um¨ u,¨ 06500 Ankara, Turkey 3 Department of Zoology, Sreegopal Banerjee College, Mogra, Hooghly, West Bengal 712148, India 4 Entomology Laboratory, Department of Zoology, University of Calcutta, 35 Ballygunge Circular Road, Kolkata, West Bengal 700019, India Correspondence should be addressed to Dinendra Raychaudhuri; [email protected] Received 25 June 2013; Accepted 7 August 2013 Academic Editors: Y. Fan and P. Simoes˜ Copyright © 2013 Sumana Saha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The present study deals with 29 lamiid species under 21 genera of Dooars, West Bengal, India. These include 4 newly recorded species, namely, Macrochenus isabellinus Aurivillius, Aesopida malasiaca Thomson, Pterolophia (Hylobrotus) lateralis Gahan and Nupserha quadrioculata (Thunberg) from India while 16 others (marked by ∙)fromthestate. 1. Introduction We (saving the second author) for nearly two decades are involved in the exploration of the long horn beetles of Subfamily Lamiinae (Cerambycidae) include members of flat the area. Present communication is one such outcome on the faced longhorn beetles that are both xylophagous and phy- lamiids dealing with 29 species under 21 genera. -

New Longhorn Beetles (Coleoptera: Cerambycidae) from Serbia
Arch. Biol. Sci., Belgrade, 57 (4), 27P-28P, 2005. NEW LONGHORN BEETLES (COLEOPTERA: CERAMBYCIDAE) FROM SERBIA. Nataša Pil1 and D. Stojanović2. 1Institute for Nature Conservation of Serbia, 21000 Novi Sad, Serbia and Montenegro, 2”Fruška Gora” National Park, 21208 Sremska Kamenica, Serbia and Montenegro UDC 597.76(497.11) Since the 1980’s, longhorn beetles (Coleoptera, Cerambycidae) They feed in the central region of the cone or occasionally in have been only randomly researched in Serbia. From earlier the base of old scales. The life cycle probably last two years, years, there are very detailed publications on this insect group and pupation very likely occurs in the soil. Adults emerge in (A d a m o v i ć , 1965; M i k š i ć and G e o r g i j e v i ć , 1971; April-July, on flowers. The given species differs from the simi- 1973; M i k š i ć and K o r p i č , 1985). lar Cortodera humeralis (Schaller, 1783) in having only sparse pubescence on the pronotum and head, with glabrous median The most recent data (I l i ć , 2005) indicate the presence line, and sparse pubescence on the outer border of the eye and of 245 longhorn beetle species (Coleoptera: Cerambycidae) in base of the antennae. Serbia. Not included in the mentioned publication, the follow- ing five species should be added to the list: Cortodera discolor 3. Vadonia hirsuta (Daniel and Daniel,1891) Fairmaire, 1866; Stenopterus similatus Holzschuh, 1979; Chlo- rophorus aegyptiacus (Fabricius, 1775); Agapanthia osmanlis (New data: Mt. -

A Comparative Histology of Male Gonads in Some Cerambycid Beetles with Notes on the Chromosomes (With 1 Plate Title and 30 Text-Figures)
A Comparative Histology of Male Gonads in Some Cerambycid Beetles with Notes on the Chromosomes (With 1 Plate Title and 30 Text-figures) Author(s) EHARA, Shôzô Citation 北海道大學理學部紀要, 12(3), 309-316 Issue Date 1956-03 Doc URL http://hdl.handle.net/2115/27161 Type bulletin (article) File Information 12(3)_P309-316.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP A Comparative Histology of Male Gonads in Some Cerambycid l Beetles with Notes on the Chromosomes ) By Sh()z() Ehara (Zoological Institute, Hokkaido University) (With 1 Plate and 30 Text-figures) Since a comparative study of the spermatogenesis in s.onie cerambycid beetles was published in 1951 by the auth.or, a considerable am.ount of data has been accumulated t.o furnish further m.orph.ol.ogical criteria for the taxonomy .of this gr.oup .of insects. In the present paper it is pr.op.osed t.o describe the c.om parative histology .of male g.onads in fifty-three species, with an additional acc.ount .on the chr.omDsomes .of twentycthree species which will supplement the histol.ogical data. Previ.otislyihe chrom.os.omes of relatedcerambycids have been reported by Stevens (1909), Snyder (1934), Smith (1950, 1953) and Yosida (1952). Before proceeding further, the author wishes to acknowledge his indebtedness to Professor Tohru Uchida for his kind guidance. His hearty thanks are also due to Professor Sajiro Makino, Drs. Eiji Momma and Tosihide H. Yosida and to Messrs. Hiroshi Nakahara and Masayasu Konishi for their valuable suggestions rendered during. the. -
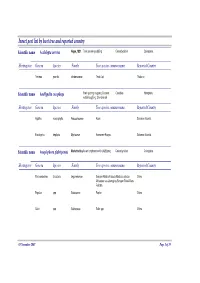
Insect Pest List by Host Tree and Reported Country
Insect pest list by host tree and reported country Scientific name Acalolepta cervina Hope, 1831 Teak canker grub|Eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Tectona grandis Verbenaceae Teak-Jati Thailand Scientific name Amblypelta cocophaga Fruit spotting bug|eng Coconut Coreidae Hemiptera nutfall bug|Eng, Chinche del Hosting tree Genera Species Family Tree species common name Reported Country Agathis macrophylla Araucariaceae Kauri Solomon Islands Eucalyptus deglupta Myrtaceae Kamarere-Bagras Solomon Islands Scientific name Anoplophora glabripennis Motschulsky Asian longhorn beetle (ALB)|eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Paraserianthes falcataria Leguminosae Sengon-Albizia-Falcata-Molucca albizia- China Moluccac sau-Jeungjing-Sengon-Batai-Mara- Falcata Populus spp. Salicaceae Poplar China Salix spp. Salicaceae Salix spp. China 05 November 2007 Page 1 of 35 Scientific name Aonidiella orientalis Newstead, Oriental scale|eng Diaspididae Homoptera 1894 Hosting tree Genera Species Family Tree species common name Reported Country Lovoa swynnertonii Meliaceae East African walnut Cameroon Azadirachta indica Meliaceae Melia indica-Neem Nigeria Scientific name Apethymus abdominalis Lepeletier, Tenthredinidae Hymenoptera 1823 Hosting tree Genera Species Family Tree species common name Reported Country Other Coniferous Other Coniferous Romania Scientific name Apriona germari Hope 1831 Long-horned beetle|eng Cerambycidae -

Coleoptera: Cerambycidae: Lamiinae)
INSECTA MUNDI, Vol. 16, No.4, December, 2002 247 Notes on Oriental Lamiini (Coleoptera: Cerambycidae: Lamiinae) Dr. Karl-Ernst Hudepohl Marktresidenz, Schillerstr. 8, 83209 Prien am Chiemsee, Germany Daniel J. Heffern 10531 Goldfield Ln. Houston, TX 77064 USA Longhorned beetles of the Tribe Lamiini have Vitticereopsius Breuning, 1961a:143 (type spe evolved into approximately 180 genera in South cies: Epepeotes vittipennis Fisher) is a synonym of East Asia and nearby regions. Many genera and Cereopsius Pascoe, 1862:344 (type species: Cere species are poorly-known and some taxa are still opsius exoletus Pascoe), new synonymy. Cereop undescribed. As a prelude to a checklist and further siusvittipennis (Fisher, 1935:599) becomes a new studies of this group, the authors propose some combination. taxonomicchangesand provide correctionsto previ Cereopsius sexmaculatus immaculithorax Bre ous literature. uning, 1974:238is not considered a valid subspecies The private collection and literature of the se- . of CereopsiussexmaculatusAurivillius, 1907:108, nior authorare depositedin Zoologische Staatssam since it is just a species with variable markings, mlung Munchen, MiinchhausenstraBe 21, D-8000, new synonymy. Munchen, Germany. Breuning (1968) placed Cyriepepeotes Breun ing, 1963: 17 (type species: Cyriepepeotes grossepunc Elongatorsidis Breuning, 1967a:183 (type spe tatus Breuning) as a synonym of Crucihammus cies: Elongatorsidis brunneus Breuning) is a syn Breuning, 1936:295 (type species: Crucihammus onym of Agniohammus Breuning, 1936:303 (type subcruciatus Breuning). Subsequently, inthemono species: Agniohammus olivaceus Breuning), new graph on Laotian Lamiinae (Rondon and Breuning, synonymy.Agniohammusbrunneus (Breuning, 1970), this generic synonymy is not listed, but the 1967a:183) becomes a new combination. appropriate combination of Crucihammus Perihammus Aurivillius, 1923:457 (type spe grossepunctatus Breuning as a Laotian species is cies: Perihammus bifasciatus Aurivillius) and Par given. -

Journal of the North-China Branch of the Royal Asiatic Society for the Year
O ~—L ^T ~~ o ?Ui JOURNAL OF THE NORTH- CHINA BRANCH OF THE BOYAL ASIATIC SOCIETY VOLUME XLIX— 1918 CONTENTS. PAGE PROCEEDINGS ix RIVER PROBLEMS IN CHINA. By Herbert Chatley, D. Sc. ... 1 SOME NOTES ON LAND-BIRDS. By H. E. Laver 13 ANIMISTIC ELEMENTS IN MOSLEM PRAYER. By Samuel M. Zwemer, F.R.G.S 38 THE EIGHT IMMORTALS OF THE TAOIST RELIGION. By Peter C. Ling 53 A CHAPTER ON FOLKLORE: I.—The Kite Festival in Foochow, China. By Lewis Hodous, D.D 76 II.— On a Method of Divination Practised at Foochow. By H. L. Harding 82 HI—Notes on the Tu T'ien Hui (fP ^ #) held at Chinkiang on the 31st May, 1917. By H. A. Ottewill 86 IV.—The Domestic Altar. By James Hutson 93 KU K'AI-CHIH'S SCROLL IN THE BRITISH MUSEUM. By J. C. Ferguson, Ph.D 101 THE THEISTIC IMPORT OF THE SUNG PHILOSOPHY. By J. P. Bruce, M.A 111 A CASE OF RITUALISM. By Evan Morgan 128 CHINESE PUZZLEDOM. By Charles Kliene, F.R.G.S. ... 144 REVIEWS OF RECENT BOOKS 160 NOTES AND QUERIES 200 ADDITIONS TO THE LIBRARY 208 LIST OF MEMBERS 211 A I: Application for Membership, stating the Name (in full) r Nationality, Profession and Address of Applicants, should be forwarded to "The SecretaE^JJorth-China Branch of the Eoyal Asiatic Society, (Shanghai^ The name should be proposed and seconded by members of the Society, but where circumstances prevent the observance of this Bule, the Council is prepared to consider applications with such references as may be given. -

An Inventory of Nepal's Insects
An Inventory of Nepal's Insects Volume III (Hemiptera, Hymenoptera, Coleoptera & Diptera) V. K. Thapa An Inventory of Nepal's Insects Volume III (Hemiptera, Hymenoptera, Coleoptera& Diptera) V.K. Thapa IUCN-The World Conservation Union 2000 Published by: IUCN Nepal Copyright: 2000. IUCN Nepal The role of the Swiss Agency for Development and Cooperation (SDC) in supporting the IUCN Nepal is gratefully acknowledged. The material in this publication may be reproduced in whole or in part and in any form for education or non-profit uses, without special permission from the copyright holder, provided acknowledgement of the source is made. IUCN Nepal would appreciate receiving a copy of any publication, which uses this publication as a source. No use of this publication may be made for resale or other commercial purposes without prior written permission of IUCN Nepal. Citation: Thapa, V.K., 2000. An Inventory of Nepal's Insects, Vol. III. IUCN Nepal, Kathmandu, xi + 475 pp. Data Processing and Design: Rabin Shrestha and Kanhaiya L. Shrestha Cover Art: From left to right: Shield bug ( Poecilocoris nepalensis), June beetle (Popilla nasuta) and Ichneumon wasp (Ichneumonidae) respectively. Source: Ms. Astrid Bjornsen, Insects of Nepal's Mid Hills poster, IUCN Nepal. ISBN: 92-9144-049 -3 Available from: IUCN Nepal P.O. Box 3923 Kathmandu, Nepal IUCN Nepal Biodiversity Publication Series aims to publish scientific information on biodiversity wealth of Nepal. Publication will appear as and when information are available and ready to publish. List of publications thus far: Series 1: An Inventory of Nepal's Insects, Vol. I. Series 2: The Rattans of Nepal. -

Forestry Department Food and Agriculture Organization of the United Nations
Forestry Department Food and Agriculture Organization of the United Nations Forest Health & Biosecurity Working Papers OVERVIEW OF FOREST PESTS THAILAND January 2007 Forest Resources Development Service Working Paper FBS/32E Forest Management Division FAO, Rome, Italy Forestry Department Overview of forest pests – Thailand DISCLAIMER The aim of this document is to give an overview of the forest pest1 situation in Thailand. It is not intended to be a comprehensive review. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. © FAO 2007 1 Pest: Any species, strain or biotype of plant, animal or pathogenic agent injurious to plants or plant products (FAO, 2004). ii Overview of forest pests – Thailand TABLE OF CONTENTS Introduction..................................................................................................................... 1 Forest pests...................................................................................................................... 1 Naturally regenerating forests..................................................................................... 1 Insects ..................................................................................................................... 1 Diseases.................................................................................................................. -

Ramesh Chander Bhagat.Pdf
Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3(12): 115-123 International Journal of Current Research in Biosciences and Plant Biology ISSN: 2349-8080 (Online) ● Volume 3 ● Number 12 (December-2016) Journal homepage: www.ijcrbp.com Original Research Article doi: http://dx.doi.org/10.20546/ijcrbp.2016.312.014 An Update on Checklist and Biodiversity of Coleopteran-fauna (Insecta) of Forestry and Mulberry Importance in Jammu and Kashmir State (India) Ramesh Chander Bhagat* P.O. Box No. 1250, G.P.O., Residency Road, Srinagar, Kashmir-190 001, J & K, India *Corresponding author. A b s t r a c t Article Info The present paper deals with a total of 64 species of beetles and weevils (Coleoptera), Accepted: 29 November 2016 belonging to 52 genera, under 14 families, associated with diverse species of forest and Available Online: 06 December 2016 mulberry plantations, occurring in vast areas and localities of Jammu and Kashmir State. The Coleopteran species of forestry and mulberry importance accounts for 73.43 K e y w o r d s % and 35.93 % respectively. The Coleopteran-fauna (47 spp.), spread over 12 families, is found to be infesting forest trees,viz. Ash, Benne, Birch, Conifers, Elms, Biodiversity Ivy, Maple, Oak, Parrotia, Plane tree, Poplars, Robinia, Salix, and Yew. Of these trees, Checklist Pines showed highest number of Coleopteran species i.e. 18, under 6 families, followed Coleopteran-fauna by Poplars, with 15 spp. (4 families) and Cedars, having 14 spp. (4 families) The Forest trees Mulberry plantations Mulberry plantations (Morus spp.) both endemic as well as exotic, have been observed to be infesting 23 spp.