CHEM 224 M+W+F Am – October 23, 2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

Chemical Kinetics HW1 (Kahn, 2010)
Chemical Kinetics HW1 (Kahn, 2010) Question 1. (6 pts) A reaction with stoichiometry A = P + 2Q was studied by monitoring the concentration of the reactant A as a function of time for eighteen minutes. The concentration determination method had a maximum error of 6 M. The following concentration profile was observed: Time (min) Conc (mM) 1 0.9850 2 0.8571 3 0.7482 4 0.6549 5 0.5885 6 0.5183 7 0.4667 8 0.4281 9 0.3864 10 0.3557 11 0.3259 12 0.3037 13 0.2706 14 0.2486 15 0.2355 16 0.2188 17 0.2111 18 0.1930 Determine the reaction order and calculate the rate constant for decomposition of A. What can be said about the mechanism or molecularity of this reaction? Question 2. (4 pts) Solve problem 2 on pg 31 in your textbook (House) using both linear and non-linear regression. Provide standard errors for the rate constant and half-life based on linear and non-linear fits. Below is the data set for your convenience: dataA = {{0, 0.5}, {10, 0.443}, {20,0.395}, {30,0.348}, {40,0.310}, {50,0.274}, {60,0.24}, {70,0.212}, {80,0.190}, {90,0.171}, {100,0.164}} Question 3. (3 pts) Solve problem 3 on pg 32 in your textbook (House). Question 4. (7 pts) The authors of the paper “Microsecond Folding of the Cold Shock Protein Measured by a Pressure-Jump Technique” suggest that the activated state of folding of CspB follows Hammond- type behavior. -

2 Reactions Observed with Alkanes Do Not Occur with Aromatic Compounds 2 (SN2 Reactions Never Occur on Sp Hybridized Carbons!)
Reactions of Aromatic Compounds Aromatic compounds are stabilized by this “aromatic stabilization” energy Due to this stabilization, normal SN2 reactions observed with alkanes do not occur with aromatic compounds 2 (SN2 reactions never occur on sp hybridized carbons!) In addition, the double bonds of the aromatic group do not behave similar to alkene reactions Aromatic Substitution While aromatic compounds do not react through addition reactions seen earlier Br Br Br2 Br2 FeBr3 Br With an appropriate catalyst, benzene will react with bromine The product is a substitution, not an addition (the bromine has substituted for a hydrogen) The product is still aromatic Electrophilic Aromatic Substitution Aromatic compounds react through a unique substitution type reaction Initially an electrophile reacts with the aromatic compound to generate an arenium ion (also called sigma complex) The arenium ion has lost aromatic stabilization (one of the carbons of the ring no longer has a conjugated p orbital) Electrophilic Aromatic Substitution In a second step, the arenium ion loses a proton to regenerate the aromatic stabilization The product is thus a substitution (the electrophile has substituted for a hydrogen) and is called an Electrophilic Aromatic Substitution Energy Profile Transition states Transition states Intermediate Potential E energy H Starting material Products E Reaction Coordinate The rate-limiting step is therefore the formation of the arenium ion The properties of this arenium ion therefore control electrophilic aromatic substitutions (just like any reaction consider the stability of the intermediate formed in the rate limiting step) 1) The rate will be faster for anything that stabilizes the arenium ion 2) The regiochemistry will be controlled by the stability of the arenium ion The properties of the arenium ion will predict the outcome of electrophilic aromatic substitution chemistry Bromination To brominate an aromatic ring need to generate an electrophilic source of bromine In practice typically add a Lewis acid (e.g. -

Reaction Mechanism Lecture 3 : Nucleophilic And
Module 10 : Reaction mechanism Lecture 3 : Nucleophilic and Electrophilic Addition and Abstraction Objectives In this lecture you will learn the following Ligand activation by metal that leads to a direct external attack at the ligand. Nucleophilic addition and nucleophilic abstraction reactions. Electrophilic addition and electrophilic abstraction reactions. The nucleophilic and electrophilic substitution and abstraction reactions can be viewed as ways of activation of substrates to allow an external reagent to directly attack the metal activated ligand without requiring prior binding of the external reagent to the metal. The attacking reagent may be a nucleophile or an electrophile. The nucleophilic attack of the external reagent is favored if the LnM fragment is a poor π−base and a good σ−acid i.e., when the complex is cationic and/or when the other metal bound ligands are electron withdrawing such that the ligand getting activated gets depleted of electron density and can undergo an external attack by a nucleophile Nu−, like LiMe or OH−. The attack of the nucleophiles may result in the formation of a bond between the nucleophiles and the activated unsaturated substrate, in which case it is called nucleophilic addition, or may result in an abstraction of a part or the whole of the activated ligand, in which case it is called the nucleophilic abstraction. The nucleophilic addition and the abstraction reactions are discussed below. Nucleophilic addition An example of a nucleophilic addition reaction is shown below. Carbon monoxide (CO) as a ligand can undergo nucleophilic attack when bound to a metal center of poor π−basicity, as the carbon center of the CO ligand is electron deficient owing to the ligand to metal σ−donation not being fully compensated by the metal to ligand π−back donation. -
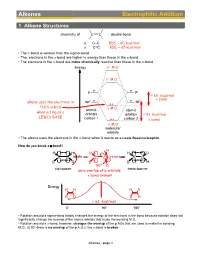
Alkenes Electrophilic Addition
Alkenes Electrophilic Addition 1 Alkene Structures chemistry of C C double bond σ C–C BDE ~ 80 kcal/mol π C=C BDE ~ 65 kcal/mol • The p-bond is weaker than the sigma-bond • The, electrons in the p-bond are higher in energy than those in the s-bond • The electrons in the p-bond are more chemically reactive than those in the s-bond Energy σ∗ M.O. π∗ M.O. p p ~ 65 kcal/mol 2 π bond alkene uses the electrons in sp2 sp THIS orbital π M.O. atomic atomic when acting as a orbitals orbitals ~ 81 kcal/mol LEWIS BASE carbon 1 carbon 2 σ bond σ M.O. molecular orbitals • The alkene uses the electrons in the p-bond when it reacts as a Lewis Base/nucleophile How do you break a p-bond? H H H H H D C C rotate C C rotate C C D D D D H 90° D cis-isomer trans-isomer zero overlap of p orbitals: π bond broken! Energy H H H D D D D H ~ 63 kcal/mol 0° 90° 180° • Rotation around a sigma-bond hardly changes the energy of the electrons in the bond because rotation does not significantly change the overlap of the atomic orbitals that make the bonding M.O. • Rotation around a p-bond, however, changes the overlap of the p AOs that are used to make the bonding M.O., at 90° there is no overlap of the p A.O.s, the p-bond is broken Alkenes : page 1 Distinguishing isomers trans- cis- • By now we are very familiar with cis- and trans-stereoisomers (diastereomers) • But what about, the following two structures, they can NOT be assigned as cis- or trans-, yet they are definitely stereoisomers (diastereomers), the directions in which their atoms point in space are different cis- / trans- Br Br We Need a different system to distinguish stereoisomers for C=C double bonds: Use Z/E notation. -

Subject Index
455 Subject Index Aminohydroxylation, 364 a-Aminoketone, 281 4-Aminophenol, 18 A Aminothiophene, 158 Abnormal Claisen rearrangement, 1 a-Aminothiophenols, 184 Acrolein, 378 Ammonium ylide, 383 2-(Acylamino)-toluenes, 245 Angeli-Rimini hydroxamic acid Acylation, 100, 145, 200 synthesis, 9 Acyl azides, 98 ~-Anomer, 225 Acylium ion, 145, 149, 175, 177 Anomeric center, 211 a-Acyloxycarboxamides, 298 Anomeric effect, 135 a-Acyloxyketones, 17 ANRORC mechanism, 10 a-Acyloxythioethers, 327 Anthracenes, 51 Acyl transfer, 17, 42, 228, 298, 305, Anti-Markovnikov addition, 219 327,345 Amdt-Eistert homologation, 11 AIBN, 22, 23, 415 Aryl-acetylene, 66 Alder ene reaction, 2 Arylation, 253 Alder's endo rule, Ill 0-Aryliminoethers, 67 Aldol condensation, 3, 14, 26, 34, 2-Arylindoles, 38 69, 130, 147, 172, 305, Aryl migration, 31 340,396,412 Autoxidation, 69, 115, 118 Aldosylamine, 8 Auwers reaction, 13 Alkyl migration, 16, 132, 315, 443 Axial, 347 Alkylation, 144, 145 Azalactone, I 00 N-Aikylation, 162 Azides, 125, 330 Alkylidene carbene, 151 Azirine, 6, 7, 281 Allan-Robinson reaction, 4, 228 Azulene, 310 Allene, 119 1t-Allyl complex, 414 B Allylation, 213, 414 Baeyer-Drewson Allylstannane, 213 indigo synthesis, 14 Allylsilanes, 349 Baeyer-Villiger oxidation, 16, 53 Alper carbonylation, 6 Baker-Venkataraman Alpine-borane®, 262 rearrangement, 17 Aluminum phenolate, 149 Balz-Schiemann reaction, 354 Amadori rearrangement, 8 Bamberger rearrangement, 18 Amide acetal, 74 Bamford-Stevens reaction, 19 Amides, 28, 67,276,339, 356 Bargellini reaction, 20 Amidine, -

Organic Seminar Abstracts
L I B RA R.Y OF THE UN IVE.R.SITY Of ILLI NOIS 547 l£6s \ 954/55 PV Return this book on or before the Latest Date stamped below. University of Illinois Library Llf.1—H41 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/organicsemi195455univ \7^ — SEMINAR TOPICS CHEMISTRY 435 I Semester 1954-55 The Quinolizinium Ion and Some of its Derivatives Harvey M. Loux, September 24 1 The Stereochemistry of Atropine and Cocaine Ho E . Knipmeyer, September 24 4 Recent Developments in the Chemistry of Cinnoline Derivatives Roger H. Kottke , October 1 8 Interpretation of Electrophiiic Aromatic Substitution and Solvolysis of Allylic and Benzhydryl Chlorides in Terms of Hyperconjugation Robert D. Stolow, October 1 11 The Decahydronaphtholic Acids and Their Relationship to the Decalols and the Decalylamines Robert J. Harder, October 8 14 Bis-Cyclopentadienyl Metal Compounds Edwin L. DeYoung, October 8 17 Ion-Pairs as Intermediates in Solvolysis Reactions Arthur H. Goldkamp, October 15 21 Synthesis of Morphine Mohan D. Nair, October 15 24 Structural and Geometrical Isomerism in the Oxidation of Azo Compounds D. F. Morrow, October 22 27 Stereochemical Aspects of Thermochromism John W. Johnson, Jr., October 22 30 A New Method for the Preparation of Olefins --The Pyrolysis of Sulfites F. M. Scheidt, October 29 33 Recent Studies of Macrocyclic Ring Systems: Cyclophanes Fred P. Hauck, Jr., October 29 36 Mechanism of the Para-Claisen Rearrangement Hugh H . Gibbs , November 5 40 A Proposed Mechanism for Basic c is -Dehydrohalogenat ion Robert M. -

Reactions of Alkenes Since Bonds Are Stronger Than Bonds, Double Bonds Tend to React to Convert the Double Bond Into Bonds
Reactions of Alkenes Since bonds are stronger than bonds, double bonds tend to react to convert the double bond into bonds This is an addition reaction. (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The bond is localized above and below the C-C bond. The electrons are relatively far away from the nuclei and are therefore loosely bound. An electrophile will attract those electrons, and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile (attacks the electrophile). Ch08 Reacns of Alkenes (landscape) Page 1 In most cases, the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: The alkene abstracts a proton from the HBr, and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product, H-Br has been added across the double bond. Ch08 Reacns of Alkenes (landscape) Page 2 Orientation of Addition Consider the addition of H-Br to 2-methylbut-2-ene: There are two possible products arising from the two different ways of adding H-Br across the double bond. But only one is observed. The observed product is the one resulting from the more stable carbocation intermediate. Tertiary carbocations are more stable than secondary. -

Electrophilic Addition Reactions.Pdf
Electrophilic Addition Reactions Electrophilic addition reactions are an important class of reactions that allow the interconversion of C=C and C≡C into a range of important functional groups. Conceptually, addition is the reverse of elimination What does the term "electrophilic addition" imply ? A electrophile, E+, is an electron poor species that will react with an electron rich species (the C=C) An addition implies that two systems combine to a single entity. Depending on the relative timing of these events, slightly different mechanisms are possible: • Reaction of the C=C with E+ to give a carbocation (or another cationic intermediate) that then reacts with a Nu- • Simultaneous formation of the two σ bonds The following pointers may aid your understanding of these reactions: • Recognise the electrophile present in the reagent combination • The electrophile adds first to the alkene, dictating the regioselectivity. • If the reaction proceeds via a planar carbocation, the reaction is not stereoselective • If the two new σ bonds form at the same time from the same species, then syn addition is observed • If the two new σ bonds form at different times from different species, then anti addition is observed Hydrogenation of Alkenes Reaction Type: Electrophilic Addition Summary • Alkenes can be reduced to alkanes with H2 in the presence of metal catalysts such as Pt, Pd, Ni or Rh. • The two new C-H σ bonds are formed simultaneously from H atoms absorbed into the metal surface. • The reaction is stereospecific giving only the syn addition product. • This reaction forms the basis of experimental "heats of hydrogenation" which can be used to establish the stability of isomeric alkenes. -

Chapter 8 - Alkenes and Alkynes II
Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes: Mechanism and Markovnikov's Rule - Hydrogen halides can add to the double bond of alkenes - The order of reactivity of the hydrogen halides in alkene addition is HI > HBr > HCl > HF - Markovnikov's rule states that in the addition of HX to an alkene, the halide atom adds to the carbon atom of the original double bond with the fewer amount of hydrogen atoms. The hydrogen of HX will bond to the carbon atom of the greater amount of hydrogen atoms. This is precisely the same thing as stabilizing a carbocation intermediate by having the nucleophile go to the more substituted carbon - More accurately, the negative portion of an unsymmetrical ionic addition reagent will bond to the carbon of the double bond with the fewer amount of hydrogen atoms while the more positive reagent will restore the stability of the carbocation - Note that this is not in water. Typically, this will happen when the solvent is not listed - A reaction is regioselective if it can potentially yield two or more constitutional isomers but produces a predominance of one 8.3 - Stereochemistry of the Ionic Addition to an Alkene - The enantiomers are produced in equal amounts because the carbocation is trigonal planar 8.4 - Addition of Sulfuric Acid to Alkenes - Adds a hydroxide group onto the alkene via Markovnikov's Rule Allyl and Benzyl Carbocations - Resonance can stabilize a product - Revised Carbocation Stability Trend: Methyl < Primary < Secondary ≈ Primary allylic ≈ Primary benzylic < Tertiary ≈ Secondary allylic ≈ Secondary benzylic < Tertiary allylic ≈ Tertiary benzylic 1 8.5 - Addition of Water to Alkenes: Acid-Catalyzed Hydration - Note how this, too, follows Markovnikov's Rule - in is the same as + because will be a strong acid (except for ), and the solvent is in HX H2O H3O HX HF larger quantities. -

6. Alkenes: Structure and Reactivity
CHEM 212 Exam 3 Part 1 Professor Kelly Boebinger 6.7 Alkene Stability Cis alkenes are less stable than trans alkenes Compare heat given off on hydrogenation: Ho Less stable isomer is higher in energy, and gives off more heat Comparing heat given off when C=C is converted to C-C More stable alkene gives off less heat Trans butene generates 5 kJ less heat than cis- butene 2 Alkene Stability Alkenes become more stable with increasing substitution Stability Order: Most to Least stable This order is due to two factors: 1) Hyperconjugation 2) Bond strengths 3 Hyperconjugation An interaction that results from the overlap of a vacant p orbital with a neighboring bond. The more the substituents present, the more opportunities for hyperconjugation. 4 Bond Strengths sp2 to sp3 is stronger than sp3-sp3 5 Problem: Which is more stable? Why? More substitution mono- vs di- & sp2-sp3 bonding Trans more stable than cis More substitution tri- vs di- 6 Writing Organic Reactions No established convention – shorthand Can be formal kinetic expression Not necessarily balanced Reactants can be before or on arrow Solvent, temperature, details, on arrow 7 6.8 Electrophilic Addition of HX to Alkenes ( X = Br, Cl, I) For unsubstituted alkenes or for symmetrically substituted alkenes, the addition of HX across a double bond appears to proceed in an identical fashion as the addition of halogens across a double bond. The reaction is successful with HCl and with HBr. H H H H ether C C + Br H H C C Br H H H H Complete the reaction: CHCHCHCH H3CH3C CHC3 -

Chem 51B Chapter 16 Notes
Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. O H • Conjugated systems are more stable than non-conjugated systems because they are resonance stabilized. • When p-orbitals overlap, the e− density in each of the π-bonds is spread over a larger volume. This lowers the energy and stabilizes the molecule. • To delocalize non-bonded electrons, or electrons in π-bonds, there must be p- orbitals that can overlap. This may mean that the hybridization of an atom is different that would have been predicted using the rules outlined in chapter 1. • Conjugated systems must be planar to allow overlap of adjacent p-orbitals. Compare: 116 A. Relative Stabilities of Conjugated Dienes Experimental heats of hydrogenation of isomeric dienes can be used to compare their relative stabilites. When hydrogenation gives the same alkane from two different dienes, the more stable diene has the smaller heat of hydrogenation Compare: Pt + H2 CH3CH2CH2CH2CH3 !H°= "61 kcal catalyst mole Pt + H CH CH CH CH CH !H°= "54 kcal 2 catalyst 3 2 2 2 3 mole Shown graphically: B. Conformations of Conjugated Dienes There are three possible stereoisomers for a conjugated diene: R R R R R R There are two possible conformations that result from free rotation about the C−C single bond (remember: double bonds cannot rotate!) s-trans conformation: Double bonds are trans about the single bond. s-cis conformation: Double bonds are cis about the single bond. 117 CH3 H H CH3 H CH3 CH3 H Most conjugated dienes prefer the s-trans conformation.