Retroviral Gene Transfer and Expression User Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rnai in Primary Cells and Difficult-To-Transfect Cell Lines
Automated High Throughput Nucleofection® RNAi in Primary Cells and Difficult-to-Transfect Cell Lines Claudia Merz, Bayer Schering Pharma AG, Berlin, Germany; Andreas Schroers, amaxa AG, Cologne, Germany; Eric Willimann, Tecan AG, Männedorf, Switzerland. Introduction Materials & Methods - Workflow Using primary cells for RNAi based applications such as target identification or – validation, requires a highly efficient transfection displaying the essential steps of the automated Nucleofector® Process: technology in combination with a reliable and robust automation system. To accomplish these requirements we integrated the amaxa 1. Transfer of the cells to the Nucleocuvette™ plate, 96-well Shuttle® in a Tecan Freedom EVO® cell transfection workstation which is based on Tecan’s Freedom EVO® liquid handling 2. Addition of the siRNA, (Steps 1 and 2 could be exchanged), platform and include all the necessary components and features for unattended cell transfection. 3. Nucleofection® process, 4. Addition of medium, Count Cells 5. Transfer of transfected cells to cell culture plate for incubation ® Nucleofector Technology prior to analysis. Remove Medium The 96-well Shuttle® combines high-throughput compatibility with the Nucleofector® Technology, which is a non-viral transfection method ideally suited for primary cells and hard-to-transfect cell lines based on a combination of buffers and electrical parameters. Nucleocuvette Plate Add Nucleofector +– The basic principle and benefits of the (empty) Solution Cell of interest Gene of interest Nucleofector® -

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Widespread Gene Transfection Into the Central Nervous System of Primates
Gene Therapy (2000) 7, 759–763 2000 Macmillan Publishers Ltd All rights reserved 0969-7128/00 $15.00 www.nature.com/gt NONVIRAL TRANSFER TECHNOLOGY RESEARCH ARTICLE Widespread gene transfection into the central nervous system of primates Y Hagihara1, Y Saitoh1, Y Kaneda2, E Kohmura1 and T Yoshimine1 1Department of Neurosurgery and 2Division of Gene Therapy Science, Department of Molecular Therapeutics, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan We attempted in vivo gene transfection into the central ner- The lacZ gene was highly expressed in the medial temporal vous system (CNS) of non-human primates using the hem- lobe, brainstem, Purkinje cells of cerebellar vermis and agglutinating virus of Japan (HVJ)-AVE liposome, a newly upper cervical cord (29.0 to 59.4% of neurons). Intrastriatal constructed anionic type liposome with a lipid composition injection of an HVJ-AVE liposome–lacZ complex made a similar to that of HIV envelopes and coated by the fusogenic focal transfection around the injection sites up to 15 mm. We envelope proteins of inactivated HVJ. HVJ-AVE liposomes conclude that the infusion of HVJ-AVE liposomes into the containing the lacZ gene were applied intrathecally through cerebrospinal fluid (CSF) space is applicable for widespread the cisterna magna of Japanese macaques. Widespread gene delivery into the CNS of large animals. Gene Therapy transgene expression was observed mainly in the neurons. (2000) 7, 759–763. Keywords: central nervous system; primates; gene therapy; HVJ liposome Introduction ever, its efficiency has not been satisfactory in vivo. Recently HVJ-AVE liposome, a new anionic-type lipo- Despite the promising results in experimental animals, some with the envelope that mimics the human immuno- gene therapy has, so far, not been successful in clinical deficiency virus (HIV), has been developed.13 Based upon 1 situations. -

Gene Therapy Glossary of Terms
GENE THERAPY GLOSSARY OF TERMS A • Phase 3: A phase of research to describe clinical trials • Allele: one of two or more alternative forms of a gene that that gather more information about a drug’s safety and arise by mutation and are found at the same place on a effectiveness by studying different populations and chromosome. different dosages and by using the drug in combination • Adeno-Associated Virus: A single stranded DNA virus that has with other drugs. These studies typically involve more not been found to cause disease in humans. This type of virus participants.7 is the most frequently used in gene therapy.1 • Phase 4: A phase of research to describe clinical trials • Adenovirus: A member of a family of viruses that can cause occurring after FDA has approved a drug for marketing. infections in the respiratory tract, eye, and gastrointestinal They include post market requirement and commitment tract. studies that are required of or agreed to by the study • Adeno-Associated Virus Vector: Adeno viruses used as sponsor. These trials gather additional information about a vehicles for genes, whose core genetic material has been drug’s safety, efficacy, or optimal use.8 removed and replaced by the FVIII- or FIX-gene • Codon: a sequence of three nucleotides in DNA or RNA • Amino Acids: building block of a protein that gives instructions to add a specific amino acid to an • Antibody: a protein produced by immune cells called B-cells elongating protein in response to a foreign molecule; acts by binding to the • CRISPR: a family of DNA sequences that can be cleaved by molecule and often making it inactive or targeting it for specific enzymes, and therefore serve as a guide to cut out destruction and insert genes. -

Horizontal Gene Transfer
Genetic Variation: The genetic substrate for natural selection Horizontal Gene Transfer Dr. Carol E. Lee, University of Wisconsin Copyright ©2020; Do not upload without permission What about organisms that do not have sexual reproduction? In prokaryotes: Horizontal gene transfer (HGT): Also termed Lateral Gene Transfer - the lateral transmission of genes between individual cells, either directly or indirectly. Could include transformation, transduction, and conjugation. This transfer of genes between organisms occurs in a manner distinct from the vertical transmission of genes from parent to offspring via sexual reproduction. These mechanisms not only generate new gene assortments, they also help move genes throughout populations and from species to species. HGT has been shown to be an important factor in the evolution of many organisms. From some basic background on prokaryotic genome architecture Smaller Population Size • Differences in genome architecture (noncoding, nonfunctional) (regulatory sequence) (transcribed sequence) General Principles • Most conserved feature of Prokaryotes is the operon • Gene Order: Prokaryotic gene order is not conserved (aside from order within the operon), whereas in Eukaryotes gene order tends to be conserved across taxa • Intron-exon genomic organization: The distinctive feature of eukaryotic genomes that sharply separates them from prokaryotic genomes is the presence of spliceosomal introns that interrupt protein-coding genes Small vs. Large Genomes 1. Compact, relatively small genomes of viruses, archaea, bacteria (typically, <10Mb), and many unicellular eukaryotes (typically, <20 Mb). In these genomes, protein-coding and RNA-coding sequences occupy most of the genomic sequence. 2. Expansive, large genomes of multicellular and some unicellular eukaryotes (typically, >100 Mb). In these genomes, the majority of the nucleotide sequence is non-coding. -

Transduction by Bacteriophage Ti * Henry Drexler
Proceedings of the National Academy of Sciences Vol. 66, No. 4, pp. 1083-1088, August 1970 Transduction by Bacteriophage Ti * Henry Drexler DEPARTMENT OF MICROBIOLOGY, BOWMAN GRAY SCHOOL OF MEDICINE, WAKE FOREST UNIVERSITY, WINSTON-SALEM, NORTH CAROLINA Communicated by Edward L. Tatum, May 25, 1970 Abstract. Amber mutants of the virulent coliphage T1 are able to transduce a wide variety of genetic characteristics from permissive to nonpermissive K strains of Escherichia coli. The virulent coliphage T1 is not known to be related to any temperate phage. Certain of the characteristics of T1 seem to be incompatible with its potential existence as a temperate phage; for example, the average latent period for T1 is only 13 min' and about 70% of its DNA is derived from the host.2 In order to demonstrate transduction by T1 it is necessray to provide condi- tions in which potential transductants are able to survive; this is accomplished by using amber mutants of T1 to transduce nonpermissive recipients. Experi- ments which show that T1 is a transducing phage are presented and have been designed chiefly to illustrate the following points: (1) Infection by T1 is able to cause heritable changes in recipients; (2) the genotype of the donor host is im- portant in determining what characteristics can be transferred to recipients; (3) the changes in the recipients are not caused by transformation; and (4) there is a similarity between the transducing activity and the plaque-forming ability of T1 with respect to serology, host range, and density. Materials and Methods. Bacterial strains: The abbreviations and symbols of Demerec et al.3 and Taylor and Trotter4 are used to describe all pertinent genotypes. -
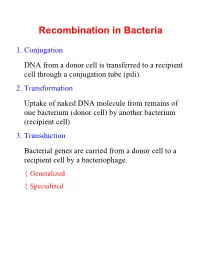
Recombination in Bacteria
Recombination in Bacteria 1. Conjugation DNA from a donor cell is transferred to a recipient cell through a conjugation tube (pili). 2. Transformation Uptake of naked DNA molecule from remains of one bacterium (donor cell) by another bacterium (recipient cell). 3. Transduction Bacterial genes are carried from a donor cell to a recipient cell by a bacteriophage. { Generalized { Specialized Conjugation Ability to conjugate located on the F-plasmid F+ Cells act as donors F- Cells act as recipients F+/F- Conjugation: { F Factor “replicates off” a single strand of DNA. { New strand goes through pili to recipient cell. { New strand is made double stranded. { If entire F-plasmid crosses, then recipient cell becomes F+, otherwise nothing happens Conjugation with Hfr Hfr cell (High Frequency Recombination) cells have F-plasmid integrated into the Chromosome. Integration into the Chromosome is unique for each F-plasmid strain. When F-plasmid material is replicated and sent across pili, Chromosomal material is included. (Figure 6.10 in Klug & Cummings) When chromosomal material is in recipient cell, recombination can occur: { Recombination is double stranded. { Donor genes are recombined into the recipient cell. { Corresponding genes from recipient cell are recombined out of the chromosome and reabsorbed by the cell. Interrupted Mating Mapping 1. Allow conjugation to start Genes closest to the origin of replication site (in the direction of replication) are moved through the pili first. 2. After a set time, interrupt conjugation Only those genes closest to the origin of replication site will conjugate. The long the time, the more that is able to conjugate. 3. -

Microbial Genetics by Dr Preeti Bajpai
Dr. Preeti Bajpai Genes: an overview ▪ A gene is the functional unit of heredity ▪ Each chromosome carry a linear array of multiple genes ▪ Each gene represents segment of DNA responsible for synthesis of RNA or protein product ▪ A gene is considered to be unit of genetic information that controls specific aspect of phenotype DNA Chromosome Gene Protein-1 Prokaryotic Courtesy: Team Shrub https://twitter.com/realscientists/status/927 cell 667237145767937 Genetic exchange within Prokaryotes The genetic exchange occurring in bacteria involve transfers of genes from one bacterium to another. The gene transfer in prokaryotic cells is thus unidirectional and the recombination events usually occur between a fragment of one chromosome (from a donor cell) and a complete chromosome (in a recipient cell) Mechanisms for genetic exchange Bacteria exchange genetic material through three different parasexual processes* namely transformation, conjugation and transduction. *Parasexual process involves recombination of genes from genetically distinct cells occurring without involvement of meiosis and fertilization Principles of Genetics-sixth edition Courtesy: Beatrice the Biologist.com by D. Peter Snustad & Michael J. Simmons (http://www.beatricebiologist.com/2014/08/bacterial-gifts/) Transformation: an introduction Transformation involves the uptake of free DNA molecules released from one bacterium (the donor cell) by another bacterium (the recipient cell). Frederick Griffith discovered transformation in Streptococcus pneumoniae (pneumococcus) in 1928. In his experiments, Griffith used two related strains of bacteria, known as R and S. The R bacteria (nonvirulent) formed colonies, or clumps of related bacteria, that Frederick Griffith 1877-1941 had a rough appearance (hence the abbreviation "R"). The S bacteria (virulent) formed colonies that were rounded and smooth (hence the abbreviation "S"). -

The Acinetobacter Baumannii Mla System and Glycerophospholipid
RESEARCH ARTICLE The Acinetobacter baumannii Mla system and glycerophospholipid transport to the outer membrane Cassandra Kamischke1, Junping Fan1, Julien Bergeron2,3, Hemantha D Kulasekara1, Zachary D Dalebroux1, Anika Burrell2, Justin M Kollman2, Samuel I Miller1,4,5* 1Department of Microbiology, University of Washington, Seattle, United States; 2Department of Biochemistry, University of Washington, Seattle, United States; 3Department of Molecular Biology and Biotechnology, The University of Sheffield, Sheffield, United Kingdom; 4Department of Genome Sciences, University of Washington, Seattle, United States; 5Department of Medicine, University of Washington, Seattle, United States Abstract The outer membrane (OM) of Gram-negative bacteria serves as a selective permeability barrier that allows entry of essential nutrients while excluding toxic compounds, including antibiotics. The OM is asymmetric and contains an outer leaflet of lipopolysaccharides (LPS) or lipooligosaccharides (LOS) and an inner leaflet of glycerophospholipids (GPL). We screened Acinetobacter baumannii transposon mutants and identified a number of mutants with OM defects, including an ABC transporter system homologous to the Mla system in E. coli. We further show that this opportunistic, antibiotic-resistant pathogen uses this multicomponent protein complex and ATP hydrolysis at the inner membrane to promote GPL export to the OM. The broad conservation of the Mla system in Gram-negative bacteria suggests the system may play a conserved role in OM biogenesis. The importance of the Mla system to Acinetobacter baumannii OM integrity and antibiotic sensitivity suggests that its components may serve as new antimicrobial *For correspondence: therapeutic targets. [email protected] DOI: https://doi.org/10.7554/eLife.40171.001 Competing interests: The authors declare that no competing interests exist. -

Lecture 3 Types, Biology and Salient Features of Vectors in Recombinant
NPTEL – Bio Technology – Genetic Engineering & Applications MODULE 1- LECTURE 3 TYPES, BIOLOGY AND SALIENT FEATURES OF VECTORS IN RECOMBINANT DNA TECHNOLOGY – PLASMID 1-3.1 Introduction: DNA molecule used for carrying an exogenous DNA into a host organism and facilitates stable integration and replication inside the host system is termed as Vector. Molecular cloning involves series of sequential steps which includes restriction digestion of DNA fragments both target DNA and vector, ligation of the target DNA with the vector and introduction into a host organism for multiplication. Then the fragments resulted after digestion with restriction enzymes are ligated to other DNA molecules that serve as vectors. In general, vectors should have following characteristics: • Capable of replicating inside the host. • Have compatible restriction site for insertion of DNA molecule (insert). • Capable of autonomous replication inside the host (ori site). • Smaller in size and able to incorporate larger insert size. • Have a selectable marker for screening of recombinant organism. 1-3.2 Plasmids: • Plasmids are naturally occurring extra chromosomal double-stranded circular DNA molecules which can autonomously replicate inside bacterial cells. Plasmids range in size from about 1.0 kb to over 250 kb. • Plasmids encode only few proteins required for their own replication (replication proteins) and these proteins encoding genes are located very close to the ori. All the other proteins required for replication, e.g. DNA polymerases, DNA ligase, helicase, etc., are provided by the host cell.Thus, only a small region surrounding Joint initiative of IITs and IISc – Funded by MHRD Page 28 of 84 NPTEL – Bio Technology – Genetic Engineering & Applications the ori site is required for replication. -

Ribozyme-Mediated, Multiplex CRISPR Gene Editing and Crispri in Plasmodium Yoelii
bioRxiv preprint doi: https://doi.org/10.1101/481416; this version posted November 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Ribozyme-Mediated, Multiplex CRISPR Gene Editing and CRISPRi in Plasmodium yoelii Michael P. Walker1 and Scott E. Lindner1 * 1Department of Biochemistry and Molecular Biology, the Huck Center for Malaria Research, Pennsylvania State University, University Park, PA. *Correspondence: Scott E. Lindner, [email protected] Running Title: CRISPR-RGR in Plasmodium yoelii Keywords: Plasmodium, CRISPR, CRISPRi, Ribozyme, HDR, ALBA bioRxiv preprint doi: https://doi.org/10.1101/481416; this version posted November 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Abstract 2 Functional characterization of genes in Plasmodium parasites often relies on genetic 3 manipulations to disrupt or modify a gene-of-interest. However, these approaches are limited by 4 the time required to generate transgenic parasites for P. falciparum and the availability of a 5 single drug selectable marker for P. yoelii. In both cases, there remains a risk of disrupting native 6 gene regulatory elements with the introduction of exogenous sequences. To address these 7 limitations, we have developed CRISPR-RGR, a SpCas9-based gene editing system for 8 Plasmodium that utilizes a Ribozyme-Guide-Ribozyme (RGR) sgRNA expression strategy. 9 Using this system with P. yoelii, we demonstrate that both gene disruptions and coding sequence 10 insertions are efficiently generated, producing marker-free and scar-free parasites with homology 11 arms as short as 80-100bp. -

Double-Stranded RNA Killer Plasmid Replication in Saccharomyces Cerevisiae (Ski Mutants/Mak Mutants) Akio TOH-E* and REED B
Proc. Natl. Acad. Sci. USA Vol. 77, No. 1, pp. 527-530, January 1980 Genetics "Superkiller" mutations suppress chromosomal mutations affecting double-stranded RNA killer plasmid replication in Saccharomyces cerevisiae (ski mutants/mak mutants) AKIo TOH-E* AND REED B. WICKNERt Laboratory of Biochemical Pharmacology, National Institute of Arthritis, Metabolism, and Digestive Diseases, National Institutes of Health, Bethesda, Maryland 20205 Communicated by G. Gilbert Ashwell, October 17,1979 ABSTRACT Saccharomyces cerevisiae strains carrying a MATERIALS AND METHODS 1.5 X 106-dalton double-stranded RNA genome in virus-like particles (killer plasmid) secrete a protein toxin that kills strains Strains. Some of the strains of Saccharomyces cerevsiae used not carrying this plasmid. At least 28 chromosomal genes (mak in this study are listed in Table 1. Description of the phenotype genes) are required to maintain or replicate this plasmid. Re- and genotype of killer strains was presented previously (21). cessive mutations in any of four other chromosomal genes (ski Curing of the killer plasmid is done by growing killer strains for superkiller) result in enhanced toxin production. We report at an elevated temperature (37°C) (23). Mitochondrial DNA that many ski- mak- double mutants are able to maintain the killer plasmid, indicating that the SKIproducts have an effect was eliminated from strains by streaking to single colonies on on plasmid replication. The skil-) mutation suppresses (by- YPAD medium containing ethidium bromide at 30 ug/ml passes) all mak mutations tested except makl6-l. A variant killer (24). plasmid is described that confers the superkiller phenotype and, Media. YPAD, YPG, SD, presporulation medium, sporula- like chromosomal ski mutations, makes several mak genes tion medium, MB medium, and various omission media were dispensable for plasmid replication.