Supporting Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Programme Young FIDE Seminar – Online Event (12 May 2021 from 9
Programme Young FIDE Seminar – Online Event (12 May 2021 from 9:45 to 13:00) Moderator: Clara van Dam (Leiden University) 9:45-10:00 Connecting and registration 10:00-10:10 Welcome and introduction into the programme by Jorrit Rijpma (Professor at Leiden University, Scientific Programme Officer of FIDE 2021) 10:10-10:40 Opening Speech by Sacha Prechal (Judge at the Court of Justice of the European Union) 10:40-10:50 Virtual coffee and tea break 10:50-12:15 Parallel sessions on the three FIDE topics Parallel session 1: National Courts and the Enforcement of EU Law – the pivotal role of national courts in the EU legal order Moderator: Maarten Schippers (Dutch Council of State) Panel members Sim Haket (Utrecht University) (Young Rapporteur) Filipe Brito Bastos (NOVA University Lisbon) Malu Beijer (Advisory Division of the Dutch Council of State) 10:50 – 11:00 Lennard Michaux (KU Leuven) 11:00 – 11:15 Panel discussion and questions 11:15 – 11:20 Virtual break 11:20 – 11:30 Giulia Gentile (Maastricht University) 11:30 – 11:45 Panel discussion and questions 11:45 – 11:50 Virtual break 11:50 – 12:00 Vincent Piegsa (Kammergericht Berlin) 12:00 – 12:15 Panel discussion and questions 1 Parallel session 2: Topic 2: Data Protection – setting global standards for the right to personal data protection Moderator: Frederik Behre (Leiden University) Panel members Teresa Quintel (University of Luxembourg) (Young Rapporteur) Michèle Fink (Max Planck Institute for Innovation and Competition) Elsbeth Beumer (Autoriteit Persoonsgegevens, the Netherlands) 10:50 -

Postgraduate Research Training in Belgium
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Lirias 1 K.U. Leuven Department of Sociology Centre for Theoretical Sociology and Sociology of Education Postgraduate Research Training in Belgium Ilse Beuselinck Jef C. Verhoeven Leuven, 1996 2 POSTGRADUATE RESEARCH TRAINING IN BELGIUM I. Beuselinck J.C. Verhoeven Postgraduate research training in Belgium is a rather recent phenomenon. Like in many West-European countries until the 1970s more attention was paid to the expansion of the universities. New universities were created in the 1960s and 1970s in order to give more youngsters the opportunity to enjoy a university education, a policy which was accompanied with more financial support for the existing universities and the expansion of scholarships for not wealthy but capable students. This development was not free from problems. Belgium is a divided country with three important cleavages which had in those years an important influence on political decisions. First, there is the religious cleavage: the split between the Roman Catholics and the anticlericals. Second, the cleavage between working classes and the propertied classes. Third, the linguistic cleavage between the Flemish and the French-speaking group. Also the university expansion was influenced by these oppositions. Belgium has in addition to state universities a strong group of free universities, mainly Catholic, but two non-confessional universities as well. Each university wanted to take as much advantage of the university expansion politics as possible. Since 1971 both systems, free and state universities are subsidized on equal basis. Moreover, the opposition between the Dutch speaking group and the French speaking group was the cause of fierce debate about the means for university expansion in Flanders, The French-speaking Community, and Brussels (Verhoeven, 1982). -

The Future of Research: Assessing the Impact of Plan S
PROMOTING The future of RESEARCH research: Assessing EXCELLENCE FOR POLICY AND the impact of Plan S PROGRESS 5TH-6TH NOVEMBER 2019 LEUVEN, BELGIUM Reception details Time and date: 17.00-19.00 Tuesday 5th November 2019 Venue: University Library, Monseigneur Ladeuzeplein 21, 3000 Leuven Conference details The bold ambition behind Plan S is to ensure that full open access to Time and date: published research finally becomes a reality. Yet, what does it mean for 09.00-16.45, the future of research? Wednesday 6th November 2019 Join us for this international event at KU Leuven, where our Venue: distinguished panels of experts will debate the prospects for The Leuven Institute researchers, universities, learned societies, academies and publishers. for Ireland in Europe, The conference is free and open to all and includes a welcome reception Janseniusstraat 1, at the historic University Library on the evening before the conference. 3000 Leuven The conference venue: The Leuven Institute for Ireland in Europe #ImpactPlanS This event is a collaboration between the Academia Europaea Cardiff Knowledge Hub, the Young Academy of Europe, KU Leuven Libraries and Cardiff University The reception venue, University Library The future of research: Assessing the impact of Plan S Speakers • President of Academia Europaea, Professor Sierd Cloetingh • Head of Open Research Policy & Partnerships at Cambridge University Press, Matthew Day © Erfgoedlabo Leuven Storygraaf • EuroDoc representative and contact for Plan S, Véronique De Herde • Pro-Vice Chancellor of Cardiff University, Please note Professor Nora de Leeuw • Your ticket includes entry to the • ALLEA Board member, Past-President Royal Irish Academy reception on the 5th as well as the and Emeritus Professor Dublin Institute for Advanced conference on the 6th. -

Devolution, Nation-Building and Development Assistance
Devolution, Nation-Building and Development Assistance: A Case Study of the Welsh Government’s Wales for Africa Programme Kathleen Mulready This thesis is submitted in fulfilment of the requirements for the degree of Doctor of Philosophy School of Geography and Planning Cardiff University 2017 Abstract Devolution, Nation-Building and Development Assistance A Case Study of the Welsh Government’s Wales for Africa programme Abstract This study explores Wales for Africa, the Welsh Government’s international development programme. It particularly considers the issues of political decentralisation, and participation in development assistance, on the making of national identity in contemporary Wales. Using a case study methodology, and a conceptual framework of the sub-state and the citizen as development actor, it explores how notions of Welsh subjectivity are tied to iterations of national identity and civic value, constructed around the concept of sustainable development, and ideas of mutual benefit and reciprocity in international development. It focuses specifically on community-based development organisations linked with partner organisations in sub- Saharan Africa. Although the potential benefits of citizen-led development initiatives to right-based approaches are recognised, little attention has previously been paid to the role of international development to sub-state nation-building. The study seeks to address this gap. Situated within the field of interpretive policy analysis, the thesis adopts a context sensitive approach focussed on how a political narrative around nationhood and civic value has been constructed around Wales’ development activities as a symbol of an alternative nation. Beginning with political devolution, the timeframe of the study ends at October 2016. -

Curriculum Vitae and Publications List
CURRICULUM VITAE AND PUBLICATIONS LIST Family (sur) name WOUTERS First names Jan Maria Florent Date and place of birth 14 July 1964, Deurne, Belgium Address and Contact Data Leuven Centre for Global Governance Studies Katholieke Universiteit Leuven (K.U.Leuven) Blijde Inkomststraat 5, 3000 Leuven, Belgium T. ++32-16-32.87.33, F. ++32-16-32.87.26 Mobile phone ++32-478.32.11.77 e-mail [email protected] Educational Qualifications PhD in Law, K.U.Leuven (1996) Visiting Researcher, Harvard Law School (1990-91) Master of Laws, Yale University (1990) Lic. Juris, Antwerp University (1987) Bachelor of Philosophy, Antwerp University (1984) Positions Currently Held Professor of International Law and the Law of International Organizations, K.U.Leuven (courses: public international law, law of international organizations, law of the World Trade Organization, armed conflicts and the law) Director of the Leuven Centre for Global Governance Studies and Institute for International Law, K.U.Leuven President, United Nations Assocation Flanders-Belgium President, Strategic Advisory Council International Flanders Of Counsel, Linklaters, Brussels Former Positions 1997-2003 Professor of European Banking and Securities Law, Maastricht University 1997-1998 Senior Lecturer on European, Economic and Financial Law, Antwerp University 1993-1998 Lecturer and Senior Lecturer in European and International Law, Maastricht University 1991-1994 Law Clerk (référendaire), European Court of Justice, Luxembourg 1989 Legal Adviser to the Belgian Minister -

KU LEUVEN Exchange Programmes: Key Data 2019
KU LEUVEN Exchange Programmes: Key data 2019 - 2020 Full legal name if the institution KU Leuven ERASMUS code B LEUVEN01 Rector Prof. Dr. Luc Sels Address Naamsestraat 22 box 5000 Postal code and town 3000 Leuven Country Belgium Website www.kuleuven.be/english Belgium’s largest and highest-ranked university and, founded in 1425, one of the oldest and most renowned universities in Europe. KU Leuven is ranked 47th in the world in THE University Ranking (2017-18) and 71st in the world by QS (2017 -18). It was ranked 1st by Reuters’ Most Innovative European University for a third year in a row in 2018. FACULTY OF ECONOMICS AND BUSINESS Dean Prof. Dr. Wilfried Lemahieu Faculty of Economics and Business Website www.feb.kuleuven.be/international www.feb.kuleuven.be/infopack Faculty of Economics and Business - Antwerp Campus Faculty of Economics and Business - Brussels Campus Faculty of Economics and Business - Leuven Campus CONTACTS Head International Office FEB Ms. Ingeborg Vandenbulcke Mobility Coordinator Dr. Dirk G. Van Waelderen Campus Leuven Campus Brussels Campus Antwerp Campus Kortrijk Outbound Ms. Barbara Dergent Ms. Suzanne Steijleman Ms. Suzanne Steijleman students Inbound Ms. Kathleen Monsieur Ms. Rebecca Rampelberg Ms. Rebecca Rampelberg students Address Faculty of Economics and Faculty of Economics and Faculty of Economics and Business Business Business Campus Leuven/Kortrijk Campus Brussels Campus Antwerp KU Leuven KU Leuven KU Leuven Naamsestraat 61 - box 3551 Warmoesberg 26 Korte Nieuwstraat 33 3000 Leuven 1000 Brussel 2000 Antwerp Belgium Belgium Belgium Telephone Outbound: +32 16 32 66 92 +32 2 210 13 37 +32 2 210 13 37 Inbound: +32 16 37 94 88 CONTACTS APPLICATION DETAILS CONTACTS Deadline nomination Once the student is nominated he/she will receive instructions via e-mail on how to complete the application. -

Research in Belgium the Story of IBA Why We Koreans • Excellent Universities
Research in Belgium The story of IBA Why we Koreans • excellent universities • similarities and common interests • But so far limited cooperation -> growth potential Why you should Belgium? Universities are very international • Founding participation in European/international networks Most innovative universities in Europe Brussels – ‘Capital of Europe’ - At the heart of Europe - More than 1,000 international companies and organizations - Open, multilingual and multicultural society - 1st most globalized country in the world - Quality of life - 16th happiest country in the world High Quality Research • 4 Nobel Prize winners for Medicine, • 3 Nobel Prize winners for Peace, • 1 for Literature, • 1 for Chemistry • 1 Nobel Prize for Physics We offer support For international researchers • Full implementation of the European Charter for researchers • Open recruitment, social security coverage, portability of grants, gender equality • Euraxess offices • Various funding opportunities Where to go? 11 universities • 4 universities in Top 200 of QS World University Rankings • Strong research-based education • Collaborations in interuniversity doctoral schools School of Arts (music, drama, plastic, performing) • Smaller size: 75 to 1,000 students per institution • Very high number of international students – 30% on average up to more than 90% Click "Header and footer" on the menu "View" and type name of the service 2 major regions Universities - Flanders- Brussels 5 universities - Ghent University - Hasselt University - KU Leuven - University of -

P-096A Phase 3 Study of Chemotherapy + Pembrolizumab Vs
abstracts Annals of Oncology (previously known as BGB-290) is a selective PARP1/2 inhibitor that crosses the blood- P À 096 A phase 3 study of chemotherapy 1 pembrolizumab vs brain barrier, has shown potent DNA–PARP trapping, and has demonstrated robust chemotherapy 1 placebo as neoadjuvant/adjuvant treatment for antitumor activity in preclinical models. In early phase clinical studies (NCT02361723; patients with gastric or gastroesophageal junction (G/GEJ) cancer: NCT03333915), pamiparib was generally well tolerated and showed promising antitu- KEYNOTE-585 - Trial in progress mor activity. These studies also established 60 mg orally twice daily as the recom- 1 2 3 4 5 6 7 mended pivotal dose. Y Bang , E Van Cutsem , C Fuchs , A Ohtsu , J Tabernero , D Ilson , W Hyung , V Strong6, T Goetze8, T Yoshikawa9, L Tang6, P Hwang10, K Shitara11 Methods: The purpose of this double-blind, placebo-controlled, randomized, multi- 1 2 center Phase 3 study (NCT03427814) conducted in Asia, Australia, Europe, and North Seoul National University Hospital, Seoul, Republic of Korea, University Hospitals Gasthuisberg Leuven and KU Leuven, Leuven, Belgium, 3Yale Cancer Center, New America is to compare the efficacy, safety, and tolerability of pamiparib with placebo as 4 5 maintenance therapy in 540 patients with advanced gastric cancer who have Haven, Connecticut, USA, National Cancer Center Hospital East, Kashiwa, Japan, Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology, Barcelona, Spain, responded to first-line, platinum-based chemotherapy. Patients who are 8 weeks after 6 7 their last platinum dose of first-line chemotherapy will be randomized 1:1 to receive Memorial Sloan Kettering Cancer Center, New York, New York, USA, Yonsei Cancer Hospital, Yonsei University Health System, Seoul, Republic of Korea, 8Institute of Clinical either pamiparib 60 mg twice daily or placebo in 28-day cycles. -
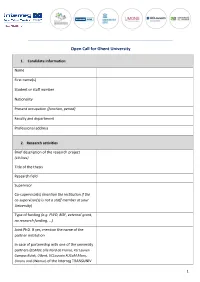
Open Call for Ghent University
Open Call for Ghent University 1. Candidate information Name First name(s) Student or staff number Nationality Present occupation (function, period) Faculty and department Professional address 2. Research activities Brief description of the research project (10 lines) Title of the thesis Research field Supervisor Co-supervisor(s) (mention the institution if the co-supervisor(s) is not a staff member at your University) Type of funding (e.g. FWO, BOF, external grant, no research funding, …) Joint PhD. If yes, mention the name of the partner institution In case of partnership with one of the university partners (CoMUE Lille Nord de France, KU Leuven Campus Kulak, UGent, UCLouvain FUCaM Mons, Umons and UNamur) of the Interreg TRANSUNIV 1 project: have you applied for the same grant in the partner university? Did you receive TRANSUNIV funding in the past? If yes, please specify (Not relevant for this first call) 3. Host institution or organisation Name, address, telephone number and e-mail of host institution Name and e-mail of contact person in host institution Give a brief description of the research stay (objectives, planning, …) (5-10 lines) Expected added-value of the stay for the research (10 lines) 4. Practical information related to research stay Duration of stay (maximum twenty working days) Estimated start and end date 5. Documents to be added A brief CV A copy of the PhD student card PhD candidate By submitting this form, I agree that the data entered above will be used by the “TRANSUNIV Interreg” partner universities to enable them to manage my application and to contact me. -

Looking Back on 55 YEARS of REWARDING EXCELLENCE
Looking back ON 55 YEARS OF REWARDING EXCELLENCE The legacy of 6 DR. ALPHONSE DE LEEUW Dr. Alphonse De Leeuw, curator of the clinical collections of the Faculty of Medicine and Pharmacy of the Univer- sité Libre de Bruxelles, died on August 24,1953. On Octo- ber 16 of that year, the Board of Directors of the NFWO accepted his legacy worth four million Belgian francs. His will determined that this legacy should be used to create a prize of 750,000 Belgian francs to be awarded once every five years. The prize, apart from his name, also bears the names of his wife Marie Damry and her former husband Leon Charles Bourlart, a cousin of Dr. De Leeuw. The prize was awarded for the first time in 1960 and the winner was chosen from a list of winners of state- awarded five-year and ten-year scientific prizes (literature, mathematics, medical sciences, ...) and winners of the Francqui prize. Three juries, with ten prominent scientists drawn from the natural sciences and medicine, mathe- matics, physics and chemistry, and the humanities, were responsible for the selection of candidates. Each jury nominated one candidate. From this shortlist the Board chose the final winner. For example, the first Dr. Damry-Bourlart Prize was awarded to Prof. Albert Dalcq, permanent secretary of the “Académie Royale de Méde- cine de Belgique”. The prize was presented on Friday, the 27th of May, 1960. From one prize TO THREE For the presentation of the second Dr. Damry-Bourlart prize, a call for prominent researchers was launched. Unlike the first prize, fellow researchers nominated the candidates. -

Code-Switching Between Structural and Sociolinguistic Perspectives Linguae & Litterae
Code-switching Between Structural and Sociolinguistic Perspectives linguae & litterae Publications of the School of Language & Literature Freiburg Institute for Advanced Studies Edited by Peter Auer, Gesa von Essen and Frick Werner Editorial Board Michel Espagne (Paris), Marino Freschi (Rom), Ekkehard König (Berlin), Michael Lackner (Erlangen-Nürnberg), Per Linell (Linköping), Angelika Linke (Zürich), Christine Maillard (Strasbourg), Lorenza Mondada (Basel), Pieter Muysken (Nijmegen), Wolfgang Raible (Freiburg), Monika Schmitz-Emans (Bochum) Volume 43 Code-switching Between Structural and Sociolinguistic Perspectives Edited by Gerald Stell and Kofi Yakpo DE GRUYTER ISBN 978-3-11-034354-0 e-ISBN (PDF) 978-3-11-034687-9 e-ISBN (EPUB) 978-3-11-038394-2 ISSN 1869-7054 Library of Congress Cataloging-in-Publication Data A CIP catalog record for this book has been applied for at the Library of Congress. Bibliographic information published by the Deutsche Nationalbibliothek The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at http://dnb.dnb.de. © 2015 Walter de Gruyter GmbH, Berlin/Munich/Boston Typesetting: Meta Systems Publishing & Printservices GmbH, Wustermark Printing and binding: Hubert & Co. GmbH & Co. KG, Göttingen ♾ Printed on acid-free paper Printed in Germany www.degruyter.com Contents Acknowledgements VII Gerald Stell, Kofi Yakpo Elusive or self-evident? Looking for common ground in approaches to code-switching 1 Part 1: Code-switching -

KU Leuven (KUL) Remained in the City of Leuven
UNIVERSITY OF LEUVEN EMA Director Websites: http://www.kuleuven.be/ Name: Prof. dr. Koen Lemmens english/ Fields of competence: International and European Human Rights Law http://www.law.kuleuven. be/faculty Other Academics Involved in EMA 2017/2018 https://www.law.kuleuve n.be/pub/en Prof. dr. Marie-Claire Foblets, Prof. dr. Paul Lemmens, Prof. dr. Stephan Department: Parmentier, Prof. dr. Jan Wouters Leuven Centre for Public The University and the City Law, Faculty of Law Leuven Contact persons : Leuven is a modern city of just over 100,000 inhabitants that has managed to Prof. dr. Koen Lemmens maintain its traditional style. It is a vibrant student city that hosts a wonderful EMA Director collection of historic buildings, from the early Romanesque period to the last Faculty of Law century. Brewing beer became an art and presently Leuven possesses the Tiensestraat 41/3441 largest brewery in Belgium. Leuven is located about 25 kilometers from 3000 Leuven Brussels, and is thus truly in the heart of Europe. Belgium Tel. +32 16 32 54 34 University of Leuven koen.lemmens@kuleuven. be Established by Pope Martinus V on 9 December 1425, the University of Leuven is the oldest and largest catholic university in the world. From the very Ms. Louise Reyntjens beginning, the university attracted students from all countries of Western Doctoral Researcher Europe. World-famous scholars such as Pope Adrian of Utrecht, Erasmus of Faculty of Law Rotterdam, Vesalius and Justus Lipsius established themselves here. ` Tiensestraat 41/3441 3000 Leuven From 1932 the University gradually became bilingual, with courses not only in Belgium French, but also in Dutch.