Natural and Anthropogenic Variations in a Channelized Water Course in Centre of Portugal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heteroptera: Gerromorpha) in Central Europe
Shortened web version University of South Bohemia in České Budějovice Faculty of Science Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe RNDr. Tomáš Ditrich Ph.D. Thesis Supervisor: Prof. RNDr. Miroslav Papáček, CSc. University of South Bohemia, Faculty of Education České Budějovice 2010 Shortened web version Ditrich, T., 2010: Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe. Ph.D. Thesis, in English. – 85 p., Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic. Annotation Ecology of Veliidae and Mesoveliidae (Hemiptera: Heteroptera: Gerromorpha) was studied in selected European species. The research of these non-gerrid semiaquatic bugs was especially focused on voltinism, overwintering with physiological consequences and wing polymorphism with dispersal pattern. Hypotheses based on data from field surveys were tested by laboratory, mesocosm and field experiments. New data on life history traits and their ecophysiological consequences are discussed in seven original research papers (four papers published in peer-reviewed journals, one paper accepted to publication, one submitted paper and one communication in a conference proceedings), creating core of this thesis. Keywords Insects, semiaquatic bugs, life history, overwintering, voltinism, dispersion, wing polymorphism. Financial support This thesis was mainly supported by grant of The Ministry of Education, Youth and Sports of the Czech Republic No. MSM 6007665801, partially by grant of the Grant Agency of the University of South Bohemia No. GAJU 6/2007/P-PřF, by The Research Council of Norway: The YGGDRASIL mobility program No. 195759/V11 and by Czech Science Foundation grant No. 206/07/0269. Shortened web version Declaration I hereby declare that I worked out this Ph.D. -

191 Distribution of Gerris Asper and G. Lateralis (Hemiptera: Heteroptera
Published December 28, 2012 Klapalekiana, 48: 191–202, 2012 ISSN 1210-6100 Distribution of Gerris asper and G. lateralis (Hemiptera: Heteroptera: Gerridae) in the Czech Republic Rozšíření Gerris asper a G. lateralis (Hemiptera: Heteroptera: Gerridae) v České republice Petr JEZIORSKI1), Petr KMENT2), Tomáš DITRICH3), Michal STRAKA4), Jan SYCHRA4) & Libor Dvořák5) 1) Na Bělidle 1, CZ-735 64 Havířov-Suchá, Czech Republic; e-mail: [email protected] 2) Department of Entomology, National Museum, kunratice 1, CZ-148 00 Praha 4, Czech Republic; e-mail: [email protected] 3) Department of Biology, Faculty of Education, University of South Bohemia, Jeronýmova 10, CZ-371 15 České Budějovice, Czech Republic; e-mail: [email protected] 4) Department of Botany and Zoology, Faculty of Science, Masaryk University, kotlářská 2, CZ-611 37 Brno, Czech Republic; e-mails: [email protected], [email protected] 5) Municipal Museum Mariánské Lázně, Goethovo náměstí 11, CZ-353 01 Mariánské Láz- ně, Czech Republic; e-mail: [email protected] Heteroptera, Gerridae, Gerris asper, Gerris lateralis, distribution, new records, Czech Republic Abstract. All the available published and unpublished distributional records of two endangered pond skaters, Gerris asper (Fieber, 1860) and Gerris lateralis Schummel, 1832 (Hemiptera: Heteroptera: Gerridae) in the Czech Republic are summarized and mapped. Gerris asper was originally described from Bohemia by Fieber (1860) without any exact locality and we have been unable to confirm its occurrence with any subsequently collected specimen. The distribution of G. asper in the Czech Republic thus seems to be limited to southern and central Moravia, with a single new record from northern Moravia, where it was found syntopically with G. -
Water Bug ID Guide
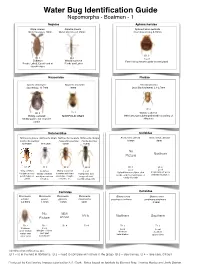
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Variations on a Theme
HENRY JOUTSIJOKI Variations on a Theme The Classification of Benthic Macroinvertebrates ACADEMIC DISSERTATION To be presented, with the permission of the board of the School of Information Sciences of the University of Tampere, for public discussion in the Auditorium Pinni B 1100, Kanslerinrinne 1, Tampere, on November 9th, 2012, at 12 o’clock. UNIVERSITY OF TAMPERE ACADEMIC DISSERTATION University of Tampere School of Information Sciences Finland Copyright ©2012 Tampere University Press and the author Distribution Tel. +358 40 190 9800 Bookshop TAJU [email protected] P.O. Box 617 www.uta.fi/taju 33014 University of Tampere http://granum.uta.fi Finland Cover design by Mikko Reinikka Acta Universitatis Tamperensis 1777 Acta Electronica Universitatis Tamperensis 1251 ISBN 978-951-44-8952-5 (print) ISBN 978-951-44-8953-2 (pdf) ISSN-L 1455-1616 ISSN 1456-954X ISSN 1455-1616 http://acta.uta.fi Tampereen Yliopistopaino Oy – Juvenes Print Tampere 2012 Abstract This thesis focused on the classification of benthic macroinvertebrates by us- ing machine learning methods. Special emphasis was placed on multi-class extensions of Support Vector Machines (SVMs). Benthic macroinvertebrates are used in biomonitoring due to their properties to react to changes in water quality. The use of benthic macroinvertebrates in biomonitoring requires a large number of collected samples. Traditionally benthic macroinvertebrates are separated and identified manually one by one from samples collected by biologists. This, however, is a time-consuming and expensive approach. By the automation of the identification process time and money would be saved and more extensive biomonitoring would be possible. The aim of the thesis was to examine what classification method would be the most appro- priate for automated benthic macroinvertebrate classification. -

A Review of the Hemiptera of Great Britain: the Aquatic and Semi-Aquatic Bugs
Natural England Commissioned Report NECR188 A review of the Hemiptera of Great Britain: The Aquatic and Semi-aquatic Bugs Dipsocoromorpha, Gerromorpha, Leptopodomorpha & Nepomorpha Species Status No.24 First published 20 November 2015 www.gov.uk/natural -england Foreword Natural England commission a range of reports from external contractors to provide evidence and advice to assist us in delivering our duties. The views in this report are those of the authors and do not necessarily represent those of Natural England. Background Making good decisions to conserve species should primarily be based upon an objective process of determining the degree of threat to the survival of a species. The recognised international approach to undertaking this is by assigning the species to one of the IUCN threat categories. This report was commissioned to update the national status of aquatic and semi-aquatic bugs using IUCN methodology for assessing threat. It covers all species of aquatic and semi-aquatic bugs (Heteroptera) in Great Britain, identifying those that are rare and/or under threat as well as non-threatened and non-native species. Reviews for other invertebrate groups will follow. Natural England Project Manager - Jon Webb, [email protected] Contractor - A.A. Cook (author) Keywords - invertebrates, red list, IUCN, status reviews, Heteroptera, aquatic bugs, shore bugs, IUCN threat categories, GB rarity status Further information This report can be downloaded from the Natural England website: www.gov.uk/government/organisations/natural-england. For information on Natural England publications contact the Natural England Enquiry Service on 0845 600 3078 or e-mail [email protected]. -

The Relative Roles of Predation and Sperm Competition on the Duration of the Post-Copulatory Association Between the Sexes in the Blue Crab, Callinectes Sapidus
Behav Ecol Sociobiol (1997) 40: 175 – 185 Springer-Verlag 1997 Paul Jivoff The relative roles of predation and sperm competition on the duration of the post-copulatory association between the sexes in the blue crab, Callinectes sapidus Received: 23 January 1996 / Accepted after revision: 9 November 1996 Abstract In many species, post-copulatory mate were in the female spermathecae, another response to guarding prevents other males from mating with the sperm competition. Larger ejaculates may need longer guarded female. In crabs, males stay with their mates to post-copulatory associations before a more effective protect the female from predators because, in some sperm plug forms. Large males stayed with the female species, mating occurs when she is soft and vulnerable longer, which is consistent with their ability to pass after molting. I tested the relative roles of sperm com- larger ejaculates than small males and suggests that there petition and predation on the duration of the post- may be costs to minimizing the duration of the post- copulatory association in the blue crab, Callinectes copulatory association. In the field, associations last sapidus. Unpaired females suffered greater predation long enough to protect the female during her vulnerable mortality than paired females and males stayed with the phase and may ensure that the guarding male fertilizes female longer in the presence of predators than in their the most eggs in the female, even if she remates. Thus, absence, suggesting that the post-copulatory association the post-copulatory association protects female blue protects females during their vulnerable period. How- crabs from additional inseminators as well as from ever, the association may also occur in blue crabs be- predators. -

The Role of Mating Systems in Sexual Selection in Parasitoid Wasps
Biol. Rev. (2014), pp. 000–000. 1 doi: 10.1111/brv.12126 Beyond sex allocation: the role of mating systems in sexual selection in parasitoid wasps Rebecca A. Boulton∗, Laura A. Collins and David M. Shuker Centre for Biological Diversity, School of Biology, University of St Andrews, Dyers Brae, Greenside place, Fife KY16 9TH, U.K. ABSTRACT Despite the diverse array of mating systems and life histories which characterise the parasitic Hymenoptera, sexual selection and sexual conflict in this taxon have been somewhat overlooked. For instance, parasitoid mating systems have typically been studied in terms of how mating structure affects sex allocation. In the past decade, however, some studies have sought to address sexual selection in the parasitoid wasps more explicitly and found that, despite the lack of obvious secondary sexual traits, sexual selection has the potential to shape a range of aspects of parasitoid reproductive behaviour and ecology. Moreover, various characteristics fundamental to the parasitoid way of life may provide innovative new ways to investigate different processes of sexual selection. The overall aim of this review therefore is to re-examine parasitoid biology with sexual selection in mind, for both parasitoid biologists and also researchers interested in sexual selection and the evolution of mating systems more generally. We will consider aspects of particular relevance that have already been well studied including local mating structure, sex allocation and sperm depletion. We go on to review what we already know about sexual selection in the parasitoid wasps and highlight areas which may prove fruitful for further investigation. In particular, sperm depletion and the costs of inbreeding under chromosomal sex determination provide novel opportunities for testing the role of direct and indirect benefits for the evolution of mate choice. -

Spatial Distribution of Semiaquatic Bugs (Heteroptera: Gerromorpha
Silva Gabreta vol. 14 (3) p. 173–178 Vimperk, 2008 Spatial distribution of semiaquatic bugs (Heteroptera: Gerromorpha) and their wing morphs in a small scale of the Pohořský Potok stream spring area (Novohradské Hory Mts.) Tomáš Ditrich1,2,*, Miroslav Papáček1 & Tomáš Broum3 1Department of Biology, Pedagogical Faculty, University of South Bohemia, Jeronýmova 10, CZ-37115 České Budějovice, Czech Republic 2Department of Ecosystem Biology, Faculty of Science, University of South Bohemia, Branišovská 31, CZ-37005 České Budějovice, Czech Republic 3Větrná 603, CZ-43151 Klášterec nad Ohří, Czech Republic *[email protected] Abstract A survey of semiaquatic bugs (Heteroptera: Gerromorpha) was managed in a small scale area (72 ha; spring area of Pohořský Potok stream, Novohradské Hory Mts., Czech Republic, Central Europe). Species compo- sition of assemblages and rates of their pteromorphs were observed in the relation to selected environmen- tal characteristics – stream velocity, site permanence, water surface coverage and site shading. Gerris gibbifer Schummel, 1832, Gerris lateralis Schummel, 1832 and Velia caprai Tamanini, 1947 are dominant gerromorphan species in the study area. Gerris species are univoltine; bivoltinism of Veliidae species can- not be excluded in this area. A redundancy analysis showed significant effect of stream velocity, site shading and site permanence on species and wing morphs composition of assemblages. Velia caprai mostly occur in shaded habitats with flowing water, whereas other species prefer still water bodies. Gerris lateralis pre- fers shaded sites as the only gerrid. Occurrence of wing morphs of the only notably wing dimorphic speci- es G. lateralis in the study area depends on site permanence. Permanent water bodies are occupied by both macropterous and apterous specimens, temporary sites are almost exclusively colonized by macropterous individuals. -

423 – Aquatic Hemiptera of Northeastern
AQUATIC HEMIPTERA OF NORTHEASTERN ALGERIA: DISTRIBUTION, PHENOLOGY AND CONSERVATION Fouzi ANNANI 1,2, Ahmed H. AL F ARHAN 3 & Boudjéma SA M RAOUI 1,3* RÉSUMÉ.— Hémiptères aquatiques du nord-est de l’Algérie : distribution, phénologie et conserva- tion.— L’échantillonnage de 83 sites à travers le complexe de zones humides du nord-est Algérien, un point chaud de la biodiversité aquatique, a permis d’identifier 35 espèces d’hémiptères aquatiques. La répartition et la phénologie des espèces sont présentées et les histoires de vie de Notonecta glauca et Notonecta obli- qua déduites. Ces deux espèces estivent dans des milieux refuges à hautes altitudes avant de redescendre se reproduire en plaine à l’automne. Diverses manifestations de changements globaux (pompage de l’eau, construction de barrages, introduction d’espèces exotiques et fragmentation des milieux) influencent néga- tivement l’intégrité écologique des milieux de la région étudiée. SUMMARY.— A survey, involving the sampling of 83 sites, investigated the aquatic hemiptera of north- eastern Algeria, a well known hotspot of aquatic biodiversity. The study recorded 35 species with data on distribution and phenology presented and discussed. Aspects of the life history of some species (Notonecta glauca and Notonecta obliqua) were inferred from their distribution and phenology and they were found to aestivate at high altitude refuges. Insect conservation in North Africa is still embryonic, relying mainly on protected areas to provide surrogate conservation to a rich and diverse group. This is inadequate in view of the current distribution of aquatic insects, often located in unprotected habitats (intermittent streams, temporary pools, dunary ponds) and the fact that diverse manifestations of global changes (loss of habitats due to water extraction and dam construction, invasive species, habitat fragmentation) are fast eroding the biodiversity of protected areas. -

Survey of Ponds at Gallows Bridge Farm Nature Reserve, Buckinghamshire
A survey of ponds at Gallows Bridge Farm Nature Reserve, Buckinghamshire A report for the Freshwater Habitats Trust Martin Hammond Ecology [email protected] September 2016 1. Introduction Gallows Bridge Farm forms part of the Upper Ray Meadows complex owned by the Berkshire, Buckinghamshire and Oxfordshire Wildlife Trust (BBOWT). Six ponds were surveyed on 19th and 20th September 2016. A range of relict stream channels and foot drains were dry at the time of the survey, as were three small ponds in the southern meadows (Ponds 16 to 18, around SP 664 198). Tetchwick Brook, a small tributary of the River Ray, was sampled briefly at three locations to provide additional records. Each pond was surveyed as per the PSYM methodology (Environment Agency, 2002), i.e. three minutes netting time divided equally between each of the meso-habitats present plus one minute examining the water surface and submerged debris. Where it was considered that additional sampling effort was warranted, additional netting was carried out but the taxa thus found were recorded separately. As far as possible, all material was identified to species level and raw data have been provided in spreadsheet format. 2. The ponds surveyed Pond Field: Main Pond (SP 669 199) The Pond Field contains around 30 ponds created by Freshwater Habitats Trust. The Main Pond is the permanent pond nearest the car park. It is a shallow, clay-bedded pond with patchy Broad-leaved Pondweed Potamogeton natans and stoneworts Chara spp., with low emergent vegetation around its banks. Nine-spined Sticklebacks are present. A total of 45 species were recorded (40 in the PSYM sample), notably including the Nationally Scarce reed-beetle Donacia thalassina. -

Organism-Substrate Relationships in Lowland Streams
H.H.Tolkamp Hature Conservation Department, Agricultural University, Wageningen Organism-substrate relationships in lowland streams IpudooI Centre for Agricultural Publishing and Documentation Wageningen - 1980 ,y* <3 "3! Communication NatureConservatio n Department211 . ISBN9 022 0075 96 Theautho r graduatedo n6 Februar y 1981a sDocto r ind e Landbouwwetenschappen atth eAgricultura l University,Wageningen ,th eNetherlands ,o na thesi swit h thesam etitl ean dcontents . ^)Centr e forAgricultura l Publishing andDocumentation ,1980 . Nopar to fthi sboo kma yb ereproduce do rpublishe d inan y form,b yprint , photoprint,microfil mo ran yothe rmean swithou twritte n permission fromth e publishers. Abstract Tolkamp, H.H. (1980)Organism-substrat e relationships inlowlan d streams.Agric . Res.Rep . (Versl.landbouwk .Onderz. )907 ,ISB N9 022 007S 96 , (xi)+ 21 1p. ,8 0 tables,4 3 figs.,31 9refs. ,Eng .an dDutc hsummaries , 14appendices . Also:Doctora l thesis,Wageningen . Afiel d and laboratory studyo n themicrodistributio n ofbotto m dwellingmacro - invertebrates toinvestigat eth erol eo fth estrea msubstrat e inth edevelopmen t andpreservatio no fth emacroinvertebrat ecommunitie s innatural ,undisturbe d low land streams isdescribed .Fiel ddat ao nbotto m substratesan d faunawer ecollecte d between 1975an d 1978fro mtw oDutc hlowlan d streams.Substrate swer e characterized by thenatur ean dth eamoun to forgani c detritus and theminera l particlesizes :i n a fieldclassificatio no n thebasi so fth evisuall y dominantparticl e sizes;i na grain-size classificationo nth ebasi so fexac t particle-size analysis inth elabora tory.Substrat epreferenc e for8 4macroinvertebrat especie swa sdemonstrate d using the Indexo fRepresentation . Substrate-selection experimentswer econducte d ina laborator y stream forthre e Trichopteraspecie s (Mioropterna sequax, Chaetopteryx villosa and Seriaostoma per sonation) andon eEphemeropter aspecie s (Ephemera daniea). -

Het News Issue 3
Issue 3 Spring 2004 Het News nd 2 Series Newsletter of the Heteroptera Recording Schemes Editorial: There is a Dutch flavour to this issue which we hope will be of interest. After all, The Netherlands is not very far as the bug flies and with a following wind there could easily be immigrants reaching our shores at any time. We have also introduced an Archive section, for historical articles, to appear when space allows. As always we are very grateful to all the providers of material for this issue and, for the next issue, look forward to hearing about your 2004 (& 2003) exploits, exciting finds, regional news, innovative gadgets etc. Sheila Brooke 18 Park Hill Toddington Dunstable Beds LU5 6AW [email protected] Bernard Nau 15 Park Hill Toddington Dunstable Beds LU5 6AW [email protected] Contents Editorial .................................................................... 1 Forthcoming & recent events ................................. 7 Dutch Bug Atlas....................................................... 1 Checklist of British water bugs .............................. 8 Recent changes in the Dutch Heteroptera............. 2 The Lygus situation............................................... 11 Uncommon Heteroptera from S. England ............. 5 Web Focus.............................................................. 12 News from the Regions ........................................... 6 From the Archives ................................................. 12 Gadget corner – Bug Mailer.....................................