Use of Changes in Fish Assemblages in the Fly River System, Papua New Guinea, to Assess Effects of the Ok Tedi Copper Mine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolutionary Genomics of a Plastic Life History Trait: Galaxias Maculatus Amphidromous and Resident Populations
EVOLUTIONARY GENOMICS OF A PLASTIC LIFE HISTORY TRAIT: GALAXIAS MACULATUS AMPHIDROMOUS AND RESIDENT POPULATIONS by María Lisette Delgado Aquije Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at Dalhousie University Halifax, Nova Scotia August 2021 Dalhousie University is located in Mi'kma'ki, the ancestral and unceded territory of the Mi'kmaq. We are all Treaty people. © Copyright by María Lisette Delgado Aquije, 2021 I dedicate this work to my parents, María and José, my brothers JR and Eduardo for their unconditional love and support and for always encouraging me to pursue my dreams, and to my grandparents Victoria, Estela, Jesús, and Pepe whose example of perseverance and hard work allowed me to reach this point. ii TABLE OF CONTENTS LIST OF TABLES ............................................................................................................ vii LIST OF FIGURES ........................................................................................................... ix ABSTRACT ...................................................................................................................... xii LIST OF ABBREVIATION USED ................................................................................ xiii ACKNOWLEDGMENTS ................................................................................................ xv CHAPTER 1. INTRODUCTION ....................................................................................... 1 1.1 Galaxias maculatus .................................................................................................. -

Nematalosa Papuensis): Implications for Freshwater Lake Management in Papua New Guinea
ResearchOnline@JCU This file is part of the following reference: Figa, Boga Soni (2014) Spatio-temporal dynamics and population biology of the Fly River Herring (Nematalosa papuensis): implications for freshwater lake management in Papua New Guinea. PhD thesis, James Cook University. Access to this file is available from: http://researchonline.jcu.edu.au/46220/ The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owner of any third party copyright material included in this document. If you believe that this is not the case, please contact [email protected] and quote http://researchonline.jcu.edu.au/46220/ Spatio-temporal dynamics and population biology of the Fly River Herring (Nematalosa papuensis): implications for freshwater lake management in Papua New Guinea. Thesis submitted by Boga Soni Figa Post Graduate Diploma of Science (JCU) Graduate Certificate in Research Methods (JCU) In August 2014 For the Degree of Doctor of Philosophy In the School of Marine and Tropical Biology James Cook University I Abstract In the face of continuous threats to the freshwater systems of the world from waste of anthropogenic origins and climate-induced environmental changes, the productivity of large floodplain ecosystems in virtually every continent is under serious threat of survival. Fish distributions and temporal dynamics are in part functions of habitat structure and conditions. Riverine fish population biology and dynamics have been studied extensively worldwide and described under various river productivity models that explain community dynamics and structure according to a range of spatial and temporal factors. Fish distribution and movements have been described in four dimensions – longitudinal, lateral, vertical, and temporal (seasonal) – that reflect the dynamic spatial and temporal nature of fish movements and habitat requirements in freshwater systems. -

Kalor J. D., Indrayani E., Akobiarek M. N. R., 2019 Fisheries Resources of Mangrove Ecosystem in Demta Gulf, Jayapura, Papua, Indonesia
Fisheries resources of mangrove ecosystem in Demta Gulf, Jayapura, Papua, Indonesia 1John D. Kalor, 2Ervina Indrayani, 3Maik N. R. Akobiarek 1 Program of Marine Sciences, Department of Marine Sciences and Fisheris, Faculty of Mathematics and Natural Sciences, University of Cenderawasih, Jayapura, Papua, Indonesia; 2 Program of Fisheries Sciences, Department of Marine Sciences and Fisheris, Faculty of Mathematics and Natural Sciences, University of Cenderawasih, Jayapura, Papua, Indonesia; 3 Program of Education Biology, Faculty of Teacher Training and Education, University of Cenderawasih, Jayapura, Papua, Indonesia. Corresponding author: J. D. Kalor, [email protected] Abstract. The mangrove ecosystem in Demta Gulf is vastly rich and distinctive, it is directly connected to the Pacific Ocean and tropical forest of the Papua Island. Mangrove ecosystems are the main ecosystems which provide ecological services such as habitat, nursery ground, feeding ground, and spawning grounds for various fish species in Demta Gulf. This study was conducted from April to June 2018 in the mangrove ecosystem of Demta Gulf, Demta District, Jayapura Regency of the Papua Province, Indonesia. Fish data were collected using gill net with 3 catching attempts/transects. There were 3 stations, 3 transect/station, and in total there were 9 transects and 27 fish-catching attempts. Mangrove data collected using transects (50 m) and plots (10 m x 10 m), there were 4 transects/stations and 3 plots/transects, in total 12 transects and 36 plots. The data were analyzed with Shannon-Wiener diversity index (H), evenness index (E), abundance analysis. Density analysis was used to analyse mangrove health status test. The result indicated that the mangrove ecosystem in Demta Gulf was categorized as healthy. -

A Biological Assessment of the Wapoga River Area of Northwestern Irian Jaya, Indonesia
Rapid Assessment Program 14 RAP Bulletin of Biological Assessment A Biological Assessment of the Wapoga River Area of Northwestern Irian Jaya, Indonesia Andrew L. Mack and Leeanne E. Alonso, Editors CENTER FOR APPLIED BIODIVERSITY SCIENCE (CABS) CONSERVATION INTERNATIONAL INDONESIAN NATIONAL INSTITUTE OF SCIENCES (LIPI) BANDUNG TECHNOLOGY INSTITUTE (ITB) UNIVERSITY OF CENDERAWASIH (UNCEN) PERLINDUNGAN DAN KONSERVASI ALAM (PKA) BADAN PENGEMBANGAN DAN PEMBANGUNAN RAP BULLETIN OF BIOLOGICAL ASSESSMENT FOURTEEN DAERAH January (BAPPEDA) 2000 1 Rapid Assessment Program 14 RAP Bulletin of Biological Assessment A Biological Assessment of the Wapoga River Area of Northwestern Irian Jaya, Indonesia Andrew L. Mack and Leeanne E. Alonso, Editors CENTER FOR APPLIED BIODIVERSITY SCIENCE (CABS) CONSERVATION INTERNATIONAL INDONESIAN NATIONAL INSTITUTE OF SCIENCES (LIPI) BANDUNG TECHNOLOGY INSTITUTE (ITB) UNIVERSITY OF CENDERAWASIH (UNCEN) PERLINDUNGAN DAN KONSERVASI ALAM (PKA) BADAN PENGEMBANGAN DAN PEMBANGUNAN RAP BULLETIN OF BIOLOGICAL ASSESSMENT FOURTEEN DAERAH January (BAPPEDA) 2000 1 RAP Bulletin of Biological Assessment is published by: Conservation International Center for Applied Biodiversity Science Department of Conservation Biology 2501 M Street NW, Suite 200 Washington, DC 20037 USA 202-429-5660 tel 202-887-0193 fax www.conservation.org Editors: Andrew L. Mack and Leeanne E. Alonso Design: Glenda P. Fábregas Map: Dan Polhemus and Leeanne E. Alonso Cover photograph: Michael Moore Translations: Iwan Wijayanto and Suer Surjadi Conservation International -
Liste Espèces
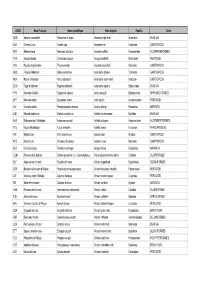
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre ODQ Anomie cascabelle Pododesmus cepio Abalone jingle shell Anomiidae BIVALVIA ABX Ormeaux nca Haliotis spp Abalones nei Haliotidae GASTROPODA REN Sébaste rose Sebastes fasciatus Acadian redfish Scorpaenidae SCORPAENIFORMES YNA Acoupa toeroe Cynoscion acoupa Acoupa weakfish Sciaenidae PERCOIDEI HSV Pourpre aiguillonnee Thais aculeata Aculeate rock shell Muricidae GASTROPODA GBQ Troque d'Adanson Gibbula adansoni Adanson's gibbula Trochidae GASTROPODA NKA Natice d'Adanson Natica adansoni Adanson's moon snail Naticidae GASTROPODA GLW Tagal d'Adanson Tagelus adansonii Adanson's tagelus Solecurtidae BIVALVIA PYD Manchot d'Adélie Pygoscelis adeliae Adelie penguin Spheniscidae SPHENISCIFORMES QFT Maconde aden Synagrops adeni Aden splitfin Acropomatidae PERCOIDEI NIV Crevette adonis Parapenaeopsis venusta Adonis shrimp Penaeidae NATANTIA DJD Modiole adriatique Modiolus adriaticus Adriatic horse mussel Mytilidae BIVALVIA AAA Esturgeon de l'Adriatique Acipenser naccarii Adriatic sturgeon Acipenseridae ACIPENSERIFORMES FCV Fucus d'Adriatique Fucus virsoides Adriatic wrack Fucaceae PHAEOPHYCEAE IRR Mitre brûlée Mitra eremitarum Adusta miter Mitridae GASTROPODA KCE Murex bruni Chicoreus brunneus Adusta murex Muricidae GASTROPODA AES Crevette ésope Pandalus montagui Aesop shrimp Pandalidae NATANTIA CGM Poisson-chat, hybride Clarias gariepinus x C. macrocephalus Africa-bighead catfish, hybrid Clariidae SILURIFORMES SUF Ange de mer africain Squatina africana African angelshark Squatinidae SQUALIFORMES -

Title 琉球列島産陸水性魚類相および文献目録 Author(S)
Title 琉球列島産陸水性魚類相および文献目録 Author(s) 吉郷, 英範 Citation Fauna Ryukyuana, 9: 1-153 Issue Date 2014-01-24 URL http://hdl.handle.net/20.500.12000/38625 Rights Fauna Ryukyuana ISSN 2187-6657 http://w3.u-ryukyu.ac.jp/naruse/lab/Fauna_Ryukyuana.html 琉球列島産陸水性魚類相および文献目録 吉郷英範 中外テクノス株式会社 〒733-0013 広島県広島市西区横川新町9-12 ([email protected]) 目次 琉球列島産陸水性魚類相および文献目録・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・1 付録 I . 琉球列島の陸水性魚類について記録された文献目録・・・・・・・・・・・・・・・・・・・・・・・・・・・・・13 付録 II. 琉球列島の陸水域から記録されている魚類目録・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・42 図版 1・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・152 要旨. 1839 年から 2013 年の間に公表された 644 2009) 際に生物標本を収集しており, 琉球列島 文献に基づいて琉球列島および大東諸島, 尖閣 における魚類に関する学術的報告 (Lay & 諸島の陸水域における魚類の分布記録を渉猟 Bennett 1839; Brevoort 1856) がそれぞれの調査 した. 二回の世界大戦中に報告のない年もあっ 記録に含められたが, 収集地域と環境が不明瞭 たが, 沖縄県の本土復帰 (1972 年) 以後に顕著 であった. その後, 琉球列島において明確な陸 に増加した. 記録の得られた島嶼は琉球列島の 水性魚類を報告したのは 1897 年の帝国博物館 33 島および魚釣島と南大東島であり, このうち の標本目録である. 1901 年からは, アメリカの 西表島と沖縄島における報告が特に多かった. Jordan を中心とした研究者らによって日本産魚 これらの文献により 27 目 110 科 678 種の魚類 類が多数報告され, これらの幾つかには琉球列 が記録されていた. このうち 334 種 (49 %) は 島の陸水性 魚類が記録された. このなかで 偶発的に陸水域に侵入した海産魚類であった Jordan & Tanaka (1927) は主に黒岩恒博士の収 が, 汽水性魚類 (143 種) と 周縁性魚類 (86 集した標本に基づいて琉球列島の淡水性魚類 種) も合わせて 34 % 含まれていた. 淡水域を を報告し, 黒岩 (1927) もその採集記録を公表 主な生息域とする純淡水魚 (59種) と通し回遊 した. Schmidt (1930, 1931) は多数の標本に基づ 魚 (56 種) は上記の種数よりもはるかに少な いて琉球列島の魚類相を報告しており, この中 かった. 琉球列島より確認された純淡水魚のほ にも数種の陸水性魚類も含まれていた. 島嶼単 とんどが外来種であり, その多くは人口が多い 位における魚類相の報告として, 岡田・木場 沖縄島を中心に記録され, その定着には人間活 (1928) や内田 (1928) による奄美大島産魚類の 動, および環境の人為的な攪乱が大きな影響を -
American Fisheries Society •
VOL 36 NO 8 AUGUST 2011 FisherieAmerican Fisheries Society • www.fisheries.orgs Lorem ipsum dolor sit amet, consectetur adipiscing elit Stream Fragmentation Thresholds for a Reproductive Guild of Great Plains Fishes Annual Report Inside Contrasting Global Game Fish and Non-Game Fish Species 03632415(2011)36(8) VOL 36 NO 8 Fisheries AUGUST 2011 Contents COLUMNS 369 PRESIDENT’S HOOK New Frontiers in Fisheries Management and Ecology: Leading the Way in a Changing World President Hubert ends his term with expressions of gratitude and a little ethical advice. Wayne A. Hubert 411 DIRECTOR’S LINE The Role of U.S. Federal Fisheries Staff in Professional Societies – Part II U.S. federal agency staff have had roadblocks in their participation in professional society affairs. It looks like now there is a serious effort by government to make it easier for them. Gus Rassam UPDATE 380 370 LEGISLATION AND POLICY Great Plains cyprinids suspected or confirmed as members of the Elden W. Hawkes, Jr. pelagic-spawning reproductive guild. FEATURE: FISH HABITAT 399 AFS 2010 ANNUAL REPORT 371 Stream Fragmentation Thresholds for a 400 Introduction Reproductive Guild of Great Plains Fishes 402 Special Projects Length of available riverine habitat between instream bar- 403 Publications riers might be a primary regulator of decline among eight 404 Awards imperiled Great Plains fishes. 406 Contributing Members Joshuah S. Perkin and Keith B. Gido 407 Donors 408 Financials FEATURE: GAME FISH 409 Meeting Planner 385 Contrasting Global Game Fish and Non-Game Fish Species 410 NEW MEMBERS On the road to developing broad strategies for the conservation and management of game fishes at a global CALENDAR scale. -

FAMILY Lutjanidae Gill 1861
FAMILY Lutjanidae Gill 1861 [=Acanthopomes, Mesopriontes, Aphareoidei, Lutjaninae, Hoplopagrinae, Platyiniini, Etelinae, Aprioninae, Paradicichthyinae, Symphorinae, Neomaenidae, Apsilinae] Notes: Name in prevailing usage, Article 35.5 Acanthopomes Duméril 1805:152 [ref. 1151] (family) ? Lutjanus [latinized to Acanthopomi by Jarocki 1822:133, 214 [ref. 4984]; no stem of the type genus, not available, Article 11.7.1.1] Mesopriontes Bleeker 1856b:40 [ref. 352] (family) Mesoprion [also as Mesopriontoidei Bleeker 1859c:52 [ref. 373] and Bleeker 1859d:19, 221 [ref. 371], and as subfamily Mesopriontiformes Bleeker 1859d:XIX [ref. 371]; senior objective synonym of Lutjaninae Gill 1861, but never used as valid after 1899] Aphareoidei Bleeker 1857:52 [ref. 356] (subfamily) Aphareus [stem Aphare- confirmed by Klunzinger 1870:767 [ref. 2621], by Jordan & Thompson 1911:440 [ref. 2540] and by Lindberg 1971:140 [ref. 27211]] Lutjaninae Gill 1861e:78 [ref. 1770] (subfamily) Lutjanus [also Gill 1862k:251 [ref. 1664]; stem sometimes seen as Lutian- or Luthian- based on Lutianus and Luthianus resp.; junior objective synonym of Mesopriontes Bleeker 1856, but that name was never used after 1899 Article 23.9.1.1, and Lutjanidae in prevailing usage, Article 23.9.1.2: Weber & de Beaufort 1936 [ref. 4606], Anderson, Jr. 1966 [ref. 7939], Kami 1973 [ref. 5616], Anderson, Jr., Kami & Johnson 1977 [ref. 115], Anderson, Jr., Talwar & Johnson 1977 [ref. 116], Randall & Guézé 1980 [ref. 8671], Bauchot, Desoutter & Allen 1981 [ref. 21549], Raj & Seeto 1983 [ref. 6817], Allen & Talbot 1985 [ref. 6491], Akazaki & Iwatsuki 1986 [ref. 6316], Allen 1986 [ref. 6208], Kishimoto, Amaoka, Kohno & Hamaguchi 1987 [ref. 6061], Potthoff, Kelley & Collins 1988 [ref. -

Metadata 2 - Data Management
Metadata 2 - Data management ANNEX 3 Correlation of Main commercial species/Commodity groups and ERS codes Revision Date Short Description of Changes 1.1 02/03/2021 Update following new ERS codes monitored 1.2 15/04/2021 Update following new ERS codes monitored ERS code Scientific name English name ISCAAP code Main commercial species CG ABM Haliotis diversicolor Small abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates AIZ Haliotis kamtschatkana Pinto abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates ABF Haliotis rufescens Red abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates ABG Haliotis gigantea Giant abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates ABJ Haliotis discus Japanese abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates ABP Haliotis midae Perlemoen abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates ABR Haliotis rubra Blacklip abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates ABX Haliotis spp Abalones nei 52 Abalone Bivalves and other molluscs and aquatic invertebrates HLT Haliotis tuberculata Tuberculate abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates SNE Concholepas concholepas False abalone 52 Abalone Bivalves and other molluscs and aquatic invertebrates ALK Gadus chalcogrammus Alaska pollock(=Walleye poll.) 32 Alaska pollock Groundfish AHC Anchovia macrolepidota Bigscale anchovy 35 Anchovy Small pelagics ANA Engraulis anchoita Argentine anchovy 35 Anchovy Small pelagics ANB Anchoa mitchilli Bay anchovy 35 Anchovy Small pelagics ANC Engraulis capensis Southern African anchovy 35 Anchovy Small pelagics ANE Engraulis encrasicolus European anchovy 35 Anchovy Small pelagics ANR Lycengraulis grossidens Atlantic sabretooth anchovy 24 Anchovy Small pelagics ANX Engraulidae Anchovies, etc. -

Lutjanus Johnii
AGE AND GROWTH THROUGH SCALE, OTOLITH, MATURATION AND FECUNDITY OF SOME COMMON SNAPPERS LUTJANUS LUTJANUS AND L. JOHNII (FAMILY LUTJANIDAE) IN PAKISTANI WATERS WITH SPECIAL REFERENCE OF DISTRIBUTION ON SINDH COAST. MUHAMMAD ASIM DEPARTMENT OF ZOOLOGY UNIVERSITY OF KARACHI Karachi-75270 CERTIFICATE UNIVERSITY OF KARACHI FACULTY OF SCIENCE This thesis entitled “AGE AND GROWTH THROUGH SCALE, OTOLITH, MATURATION AND FECUNDITY OF COMMON SNAPPERS LUTJANUS LUTJANUS AND LUTJANUS JOHNII (FAMILY LUTJANIDAE) IN PAKISTANI WATERS WITH SPECIAL REFERENCE TO DISTRIBUTION ON SINDH COAST” .Submitted by Muhammad Asim is accepted by the Department of Zoology, University of Karachi, as satisfying the thesis requirement for the degree of Doctor of Philosophy (Ph.D.) in Zoology. Supervisor ____________________________ Prof. Dr. Habib-ul-Hassan Department of Zoology University of Karachi. Co- supervisor ____________________________ Prof. Dr. M. Zaheer Khan Department of Zoology University of Karachi. This Dissertation is dedicated to my wife, beautiful son Muhammad Hamdan Asim my sisters Mrs. Nafees Subohi (Late) and Ms Anees Roohi who always wishes and prays for my success. ACKNOWLEDGMENT My deep reverence goes to Almighty Allah, my supreme, who enabled me to complete the present work. I thankful my respected supervisor Prof.Dr. Habib-ul-Hassan and co-supervisor Prof. Dr. Muahmmad Zaheer Khan for their support, guidance and encouragement. I would like to thanks Dr. Constantine Stamatppoulos (Fisheries Resources Monitoring and Assessment Lamans) ICLARM for the guidance to determine the age and growth by von- Bertalanffy growth parameters by VONBIT software tool. I am also acknowledged to Respected Muhammad Moazzam (Ex. Director General, Marine Fisheries Department Government of Pakistan and present Technical Advisor (Marine Fisheries) WWF-Pakistan for his guidance and encouragements. -

Global Participation in and Public Attitudes Toward Recreational Fishing: International Perspectives and Developments
Reviews in Fisheries Science & Aquaculture ISSN: 2330-8249 (Print) 2330-8257 (Online) Journal homepage: https://www.tandfonline.com/loi/brfs21 Global Participation in and Public Attitudes Toward Recreational Fishing: International Perspectives and Developments Robert Arlinghaus, Øystein Aas, Josep Alós, Ivan Arismendi, Shannon Bower, Steven Carle, Tomasz Czarkowski, Kátia M. F. Freire, John Hu, Len M. Hunt, Roman Lyach, Andrzej Kapusta, Pekka Salmi, Alexander Schwab, Jun-ichi Tsuboi, Marek Trella, Daryl McPhee, Warren Potts, Arkadiusz Wołos & Zi- Jiang Yang To cite this article: Robert Arlinghaus, Øystein Aas, Josep Alós, Ivan Arismendi, Shannon Bower, Steven Carle, Tomasz Czarkowski, Kátia M. F. Freire, John Hu, Len M. Hunt, Roman Lyach, Andrzej Kapusta, Pekka Salmi, Alexander Schwab, Jun-ichi Tsuboi, Marek Trella, Daryl McPhee, Warren Potts, Arkadiusz Wołos & Zi-Jiang Yang (2020): Global Participation in and Public Attitudes Toward Recreational Fishing: International Perspectives and Developments, Reviews in Fisheries Science & Aquaculture, DOI: 10.1080/23308249.2020.1782340 To link to this article: https://doi.org/10.1080/23308249.2020.1782340 Published online: 29 Jun 2020. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=brfs21 REVIEWS IN FISHERIES SCIENCE & AQUACULTURE https://doi.org/10.1080/23308249.2020.1782340 REVIEW Global Participation in and Public Attitudes Toward Recreational -

Global Participation in and Public Attitudes Toward Recreational Fishing: International Perspectives and Developments
Reviews in Fisheries Science & Aquaculture ISSN: 2330-8249 (Print) 2330-8257 (Online) Journal homepage: https://www.tandfonline.com/loi/brfs21 Global Participation in and Public Attitudes Toward Recreational Fishing: International Perspectives and Developments Robert Arlinghaus, Øystein Aas, Josep Alós, Ivan Arismendi, Shannon Bower, Steven Carle, Tomasz Czarkowski, Kátia M. F. Freire, John Hu, Len M. Hunt, Roman Lyach, Andrzej Kapusta, Pekka Salmi, Alexander Schwab, Jun-ichi Tsuboi, Marek Trella, Daryl McPhee, Warren Potts, Arkadiusz Wołos & Zi- Jiang Yang To cite this article: Robert Arlinghaus, Øystein Aas, Josep Alós, Ivan Arismendi, Shannon Bower, Steven Carle, Tomasz Czarkowski, Kátia M. F. Freire, John Hu, Len M. Hunt, Roman Lyach, Andrzej Kapusta, Pekka Salmi, Alexander Schwab, Jun-ichi Tsuboi, Marek Trella, Daryl McPhee, Warren Potts, Arkadiusz Wołos & Zi-Jiang Yang (2020): Global Participation in and Public Attitudes Toward Recreational Fishing: International Perspectives and Developments, Reviews in Fisheries Science & Aquaculture, DOI: 10.1080/23308249.2020.1782340 To link to this article: https://doi.org/10.1080/23308249.2020.1782340 Published online: 29 Jun 2020. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=brfs21 REVIEWS IN FISHERIES SCIENCE & AQUACULTURE https://doi.org/10.1080/23308249.2020.1782340 REVIEW Global Participation in and Public Attitudes Toward Recreational