Exon-Trapping Assay Improves Clinical Interpretation of COL11A1 and COL11A2 Intronic Variants in Stickler Syndrome Type 2 and Otospondylomegaepiphyseal Dysplasia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetics of Congenital Hand Anomalies
G. C. Schwabe1 S. Mundlos2 Genetics of Congenital Hand Anomalies Die Genetik angeborener Handfehlbildungen Original Article Abstract Zusammenfassung Congenital limb malformations exhibit a wide spectrum of phe- Angeborene Handfehlbildungen sind durch ein breites Spektrum notypic manifestations and may occur as an isolated malforma- an phänotypischen Manifestationen gekennzeichnet. Sie treten tion and as part of a syndrome. They are individually rare, but als isolierte Malformation oder als Teil verschiedener Syndrome due to their overall frequency and severity they are of clinical auf. Die einzelnen Formen kongenitaler Handfehlbildungen sind relevance. In recent years, increasing knowledge of the molecu- selten, besitzen aber aufgrund ihrer Häufigkeit insgesamt und lar basis of embryonic development has significantly enhanced der hohen Belastung für Betroffene erhebliche klinische Rele- our understanding of congenital limb malformations. In addi- vanz. Die fortschreitende Erkenntnis über die molekularen Me- tion, genetic studies have revealed the molecular basis of an in- chanismen der Embryonalentwicklung haben in den letzten Jah- creasing number of conditions with primary or secondary limb ren wesentlich dazu beigetragen, die genetischen Ursachen kon- involvement. The molecular findings have led to a regrouping of genitaler Malformationen besser zu verstehen. Der hohe Grad an malformations in genetic terms. However, the establishment of phänotypischer Variabilität kongenitaler Handfehlbildungen er- precise genotype-phenotype correlations for limb malforma- schwert jedoch eine Etablierung präziser Genotyp-Phänotyp- tions is difficult due to the high degree of phenotypic variability. Korrelationen. In diesem Übersichtsartikel präsentieren wir das We present an overview of congenital limb malformations based Spektrum kongenitaler Malformationen, basierend auf einer ent- 85 on an anatomic and genetic concept reflecting recent molecular wicklungsbiologischen, anatomischen und genetischen Klassifi- and developmental insights. -

Orphanet Journal of Rare Diseases Biomed Central
Orphanet Journal of Rare Diseases BioMed Central Review Open Access Brachydactyly Samia A Temtamy* and Mona S Aglan Address: Department of Clinical Genetics, Human Genetics and Genome Research Division, National Research Centre (NRC), El-Buhouth St., Dokki, 12311, Cairo, Egypt Email: Samia A Temtamy* - [email protected]; Mona S Aglan - [email protected] * Corresponding author Published: 13 June 2008 Received: 4 April 2008 Accepted: 13 June 2008 Orphanet Journal of Rare Diseases 2008, 3:15 doi:10.1186/1750-1172-3-15 This article is available from: http://www.ojrd.com/content/3/1/15 © 2008 Temtamy and Aglan; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Brachydactyly ("short digits") is a general term that refers to disproportionately short fingers and toes, and forms part of the group of limb malformations characterized by bone dysostosis. The various types of isolated brachydactyly are rare, except for types A3 and D. Brachydactyly can occur either as an isolated malformation or as a part of a complex malformation syndrome. To date, many different forms of brachydactyly have been identified. Some forms also result in short stature. In isolated brachydactyly, subtle changes elsewhere may be present. Brachydactyly may also be accompanied by other hand malformations, such as syndactyly, polydactyly, reduction defects, or symphalangism. For the majority of isolated brachydactylies and some syndromic forms of brachydactyly, the causative gene defect has been identified. -

Modeling Congenital Disease and Inborn Errors of Development in Drosophila Melanogaster Matthew J
© 2016. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2016) 9, 253-269 doi:10.1242/dmm.023564 REVIEW SUBJECT COLLECTION: TRANSLATIONAL IMPACT OF DROSOPHILA Modeling congenital disease and inborn errors of development in Drosophila melanogaster Matthew J. Moulton and Anthea Letsou* ABSTRACT particularly difficult to manage clinically {e.g. CHARGE syndrome Fly models that faithfully recapitulate various aspects of human manifesting coloboma [emboldened words and phrases are defined disease and human health-related biology are being used for in the glossary (see Box 1)], heart defects, choanal atresia, growth research into disease diagnosis and prevention. Established and retardation, genitourinary malformation and ear abnormalities new genetic strategies in Drosophila have yielded numerous (Hsu et al., 2014), and velocardiofacial or Shprinstzens syndrome substantial successes in modeling congenital disorders or inborn manifesting cardiac anomaly, velopharyngeal insufficiency, errors of human development, as well as neurodegenerative disease aberrant calcium metabolism and immune dysfunction (Chinnadurai and cancer. Moreover, although our ability to generate sequence and Goudy, 2012)}. Estimates suggest that the cause of at least 50% of datasets continues to outpace our ability to analyze these datasets, congenital abnormalities remains unknown (Lobo and Zhaurova, the development of high-throughput analysis platforms in Drosophila 2008). It is vital that we understand the etiology of congenital has provided access through the bottleneck in the identification of anomalies because this knowledge provides a foundation for improved disease gene candidates. In this Review, we describe both the diagnostics as well as the design of preventatives and therapeutics that traditional and newer methods that are facilitating the incorporation of can effectively alleviate or abolish the effects of disease. -
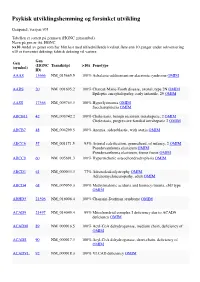
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Table of Contents
Table of contents Acknowledgements I List of Figures III List of Tables XI List of Abbreviations XIV Summary XVI Part 1 Primary microcephaly 1 INTRODUCTION 1 1.1 Primary microcephaly (MCPH) 2 1.2 Structure of cerebral cortex 5 1.3 Development of cerebral cortex 7 1.4 Clinical features of primary microcephaly 8 1.5 MCPH genes and their function 10 1.5.1 Microcephalin (MCPH1) 10 1.5.2 Abnormal spindle like microcephaly associated (ASPM) 11 1.5.3 Cyclin dependent kinase5 regulatory associated protein (CDK5RAP2) 12 1.5.4 Centrosomal protein J (CENPJ) 13 1.5.5 STIL (SCL/TAL1 interrupting locus) 13 1.6 Objectives of study 16 2 MATERIALS AND METHODS 17 2.1 Families studied 17 2.1.1 MCP3 18 2.1. 2 MCP6 19 2.1.3 MCP7 20 2.1.4 MCP9 21 2.1.5 MCP11 22 2.1.6 MCP15 23 2.1. 7 MCP17 24 2.1.8 MCP18 25 2.1.9 MCP21 26 2.1.10 MCP22 27 2.1.11 MCP35 28 2.1.12 MCP36 29 2.2 Clinical data 29 2.3 Blood sampling 32 2.4 DNA extraction 33 2.5 Polymerase chain reaction 33 2.6 Agarose gel electrophoresis 33 2.7 Genotyping using fluorescently labeled primers 34 2.8 Preparation of samples for ABI 3130xl genetic analyzer 35 2.9 Linkage analysis 35 2.10 Mutation screening 36 2.10.1 DNA sequencing 36 2.10.2 Purification of PCR product from agarose gel 37 2.10.3 Purification of PCR product 37 2.10.4 Sequencing PCR reactions 37 2.10.5 Precipitation of sequencing PCR products 38 2.10.6 Sequencing data analysis 38 2.11 Restriction analysis 39 3 RESULTS 51 3.1 Linkage studies 51 3.2 Mutation analysis 52 3.2.1 Sequencing of ASPM in MCPH5 linked families 52 3.2.2 Compound heterozygous -

Daniel Lopo Polla.Pdf
Pró-Reitoria de Pós-Graduação e Pesquisa Programa de Pós-Graduação Stricto Sensu em Ciências Genômicas e Biotecnologia SEQUENCIAMENTO DE EXOMA PARCIAL COMO FERRAMENTA DE DIAGNÓSTICO MOLECULAR DE DISPLASIAS ESQUELÉTICAS Autor: Daniel Lôpo Polla Orientador: Dr. Robert Pogue Coorientador: Dr. Rinaldo Wellerson Pereira Brasília - DF 2014 DANIEL LÔPO POLLA SEQUENCIAMENTO DE EXOMA PARCIAL COMO FERRAMENTA DE DIAGNÓSTICO MOLECULAR DE DISPLASIAS ESQUELÉTICAS Dissertação apresentada ao Programa de Pós-graduação Stricto Sensu em Ciências Genômicas e Biotecnologia da Universidade Católica de Brasília, como requisito parcial para a obtenção do Título de Mestre em Ciências Genômicas e Biotecnologia. Orientador: Dr. Robert Pogue Coorientador: Dr. Rinaldo Wellerson Pereira Brasília 2014 P771s Polla, Daniel Lôpo. Sequenciamento de exoma parcial como ferramenta de diagnóstico molecular de displasias esqueléticas. / Daniel Lôpo Polla – 2014. 133 f.; il : 30 cm Dissertação (mestrado) – Universidade Católica de Brasília, 2014. Orientação: Prof. Dr. Robert Pogue Coorientação: Prof. Dr. Rinaldo Wellerson Pereira 1. Biotecnologia. 2. Diagnóstico molecular. 3. Displasia esquelética. I. Pogue, Robert, orient. II. Pereira, Rinaldo Wllerson, coorient. III. Título. CDU 577.21 Ficha elaborada pela Biblioteca Pós-Graduação da UCB Dissertação de autoria de Daniel Lôpo Polla, intitulada “SEQUENCIAMENTO DE EXOMA PARCIAL COMO FERRAMENTA DE DIAGNÓSTICO MOLECULAR DE DISPLASIAS ESQUELÉTICAS”, apresentada como requisito parcial para a obtenção de grau de Mestre em Ciências Genômicas e Biotecnologia da Universidade Católica de Brasília, em 12 de março de 2014, defendida e aprovada pela banca examinadora abaixo assinada: Brasília 2014 AGRADECIMENTOS Agradeço em primeiro lugar a Deus que iluminou o meu caminho durante mais esta caminhada, dando-me força e paciência para superar os desafios. -

Skeletal Dysplasia Panel Versie V1 (345 Genen) Centrum Voor Medische Genetica Gent
H9.1-OP2-B40: Genpanel Skeletal dysplasia, V1, in voege op 14/02/2020 Skeletal_dysplasia panel versie V1 (345 genen) Centrum voor Medische Genetica Gent Associated phenotype, OMIM phenotype ID, phenotype Gene OMIM gene ID mapping key and inheritance pattern Atrial fibrillation, familial, 12, 614050 (3), Autosomal dominant; ABCC9 601439 Cardiomyopathy, dilated, 1O, 608569 (3); Hypertrichotic osteochondrodysplasia, 239850 (3), Autosomal dominant Congenital heart defects and skeletal malformations syndrome, 617602 (3), Autosomal dominant; Leukemia, Philadelphia ABL1 189980 chromosome-positive, resistant to imatinib, 608232 (3), Somatic mutation Short stature and advanced bone age, with or without early-onset osteoarthritis and/or osteochondritis dissecans, 165800 (3), ACAN 155760 Autosomal dominant; Spondyloepimetaphyseal dysplasia, aggrecan type, 612813 (3), Autosomal recessive; ?Spondyloepiphyseal dysplasia, Kimberley type, 608361 (3), Autosomal dominant Spondyloenchondrodysplasia with immune dysregulation, 607944 ACP5 171640 (3), Autosomal recessive Fibrodysplasia ossificans progressiva, 135100 (3), Autosomal ACVR1 102576 dominant Weill-Marchesani syndrome 1, recessive, 277600 (3), Autosomal ADAMTS10 608990 recessive Weill-Marchesani 4 syndrome, recessive, 613195 (3), Autosomal ADAMTS17 607511 recessive ADAMTSL2 612277 Geleophysic dysplasia 1, 231050 (3), Autosomal recessive AFF4 604417 CHOPS syndrome, 616368 (3), Autosomal dominant AGA 613228 Aspartylglucosaminuria, 208400 (3), Autosomal recessive Rhizomelic chondrodysplasia punctata, -

Skeletal Dysplasia Panel Versie V2 (346 Genen) Centrum Voor Medische Genetica Gent
H9.1-OP2-B40: Genpanel Skeletal dysplasia, V2, in voege op 26/05/2020 Skeletal_dysplasia panel versie V2 (346 genen) Centrum voor Medische Genetica Gent Associated phenotype, OMIM phenotype ID, phenotype Gene OMIM gene ID mapping key and inheritance pattern Atrial fibrillation, familial, 12, 614050 (3), Autosomal dominant; ABCC9 601439 Cardiomyopathy, dilated, 1O, 608569 (3); Hypertrichotic osteochondrodysplasia, 239850 (3), Autosomal dominant Congenital heart defects and skeletal malformations syndrome, 617602 (3), Autosomal dominant; Leukemia, Philadelphia ABL1 189980 chromosome-positive, resistant to imatinib, 608232 (3), Somatic mutation Short stature and advanced bone age, with or without early-onset osteoarthritis and/or osteochondritis dissecans, 165800 (3), ACAN 155760 Autosomal dominant; Spondyloepimetaphyseal dysplasia, aggrecan type, 612813 (3), Autosomal recessive; ?Spondyloepiphyseal dysplasia, Kimberley type, 608361 (3), Autosomal dominant Spondyloenchondrodysplasia with immune dysregulation, 607944 (3), ACP5 171640 Autosomal recessive ACVR1 102576 Fibrodysplasia ossificans progressiva, 135100 (3), Autosomal dominant Weill-Marchesani syndrome 1, recessive, 277600 (3), Autosomal ADAMTS10 608990 recessive Weill-Marchesani 4 syndrome, recessive, 613195 (3), Autosomal ADAMTS17 607511 recessive ADAMTSL2 612277 Geleophysic dysplasia 1, 231050 (3), Autosomal recessive AFF4 604417 CHOPS syndrome, 616368 (3), Autosomal dominant AGA 613228 Aspartylglucosaminuria, 208400 (3), Autosomal recessive Rhizomelic chondrodysplasia punctata, -

1 – Ust-Dzhegutinsky District; 2
Supplementary Tables S1–S6 Note: 1 – Ust-Dzhegutinsky district; 2 – Karachaevsky district; 3 – Malokarachaevsky district; 4– Cherkessk City; 5 – Prikubansky district; 6 – Urupsky district; 7 – Zelenchuksky district; 8 – Abazinsky district; 9 – Khabezsky district; 10 – Adyge-Khablsky district; 11 – Nogaysky district; T/I – type of inheritance; AD – autosomal dominant type of inheritance, AR – autosomal recessive type of inheritance, XL – X-linked type of inheritance. PS – Phenotypic Series for OMIM in case of heterogeneity of the disease; Isol. – Isolated cases; Som. – Somatic mutation Table S1. Nosological spectrum and prevalence (per 100000) of hereditary neurological diseases in Karachay-Cherkess Republic (KChR) Number of patients Prevalence (per 100000) 1 2 3 4 5 6 7 8 9 10 11 Σ Kara Russi Circ Aba Nog Other Σ № ОМIМ Diagnosis T/I chays an assia zins ais s ns 1. #156200 Undifferentiated mental retardation АD 7 4 4 7 4 6 7 39 12.31 6.68 15.7 13.6 9.50 2. #249500 Undifferentiated mental retardation AR 10 10 19 6 23 9 1 2 17 16 20 133 31.40 26.71 41.2 9.02 74.6 76.68 32.41 3. #309530 Undifferentiated mental retardation XL 10 8 3 6 18 5 6 3 12 2 3 76 40.63 26.71 19.7 42.1 54.3 125.5 37.04 4. #300624 Martin-Bell syndrome XL 2 1 4 3 2 2 1 15 8.62 7.87 36.1 7.31 5. #158600 Spinal muscular atrophy, juvenile, proximal АD 2 2 1.48 0.49 6. #253300 Spinal muscular atrophy, type 1 AR 1 1 2 0.62 3.01 0.49 7. -

Textbooks and Monographs
Appendix A Textbooks and Monographs Beighton P (1993) McKusick's heritable disorders of connective tissue, 5th edn. CV Mosby, St Louis, MO Beighton P (1988) Inherited disorders of the skeleton, 2nd edn. Churchill Livingstone, Edinburgh Beighton P, Cremin BJ (1980) Sclerosing bone dysplasias. Springer, Berlin Beighton P, Grahame R, Bird H (1999) Hypermobility of joints, 3rd edn. Springer, Berlin Buyse ML (ed) (1990) Birth defects encyclopaedia. Blackwell Scientific, Cam bridge, MA Connor JM (1983) Soft tissue ossification. Springer, Berlin Cremin BJ, Beighton P (1978) Bone dysplasias in infancy. A radiological atlas. Springer, Berlin Donnai D, Winter RM (1995) Congenital malformation syndromes. Chapman & Hall, London Gorlin RJ, Cohen MM, Levin LS (1990) Syndromes of the head and neck, 3rd edn. McGraw-Hill, New York Horan F, Beighton P (1982) Orthopaedic problems in inherited skeletal disorders. Springer, Berlin Jones KL (1997) Smith's recognisable patterns of human malformation, 5th edn. WB Saunders, Philadelphia, P A 234 Gamut Index of Skeletal Dysplasias Kaufman JH (ed) (1973) Intrinsic diseases of bone. Progress in Pediatric Radiology, vol 4. Karger, Basel Maroteaux P (1979) Bone diseases of children. Lippincott, Philadelphia, PA McKusick VA (1998) Mendelian inheritance in man, 12th edn. Johns Hopkins Press, Baltimore, MA Mueller RF, Young ID (1998) Emery's elements of medical genetics. Harcourt Brace, London Papadatos CJ, Bartsocas CS (eds) (1982) Skeletal dysplasias. Progress in clinical and biological research, vol 104. Alan R Liss, New York Poznanski AK (1974) The hand in radiologic diagnosis. WB Saunders, Philadelphia, PA Royce PM, Steinmann B (1993) Connective tissue and its heritable disorders. Wiley-Liss, New York Spranger JW, Langer LO, Wiedemann HR (1974) Bone dysplasias. -

Holt-Oram Syndrome
Holt-Oram Syndrome In 1960, Holt and Oram described a family in which nine b. Unilateral or bilateral members in four generations were affected by skeletal abnor- c. Symmetric or asymmetric (left side more affected malities of the upper limbs, cardiac malformations, and than the right side) arrhythmias. The prevalence is about 1 in 100,000 live births. d. Severe (phocomelia) to mild phenotype due to aplasia, About 40 to 85% of cases are due to fresh mutation. hypoplasia, fusion, or anomalous development of involved bones e. Thumbs GENETICS/BASIC DEFECTS i. Aplasia 1. An autosomal dominant disorder ii. Hypoplasia a. Complete penetrance iii. Triphalangeal b. Widely variable expression iv. Syndactyly 2. Holt-Oram syndrome frequently linked to the gene TBX5, v. Long which is mapped to 12q24.1 vi. Normal 3. Caused by mutations in the TBX5 gene, a member of the f. Fingers T-box family that encodes a transcription factor i. Clinodactyly a. Mutations predicted to create null alleles cause sub- ii. Brachydactyly stantial abnormalities in both the limb and heart iii. Hypoplasia b. Nonsense mutations of TBX5 produce distinct pheno- iv. Absent types v. Syndactyly i. One class of missense mutations: causes signifi- vi. Normal cant cardiac malformations but only minor g. Radial bones skeletal abnormalities i. Hypoplasia ii. Other class of nonsense mutations: causes exten- ii. Aplasia sive upper limb malformations but less signifi- h. Ulnar cant cardiac abnormalities i. Hypoplasia 4. Intrafamilial variations of the malformations: suggest that ii. Aplasia genetic background or modifier genes plays an important i. Carpal bones role in the phenotypic expression of Holt-Oram syndrome i. -

Fork Stalling and Template Switching As a Mechanism for Polyalanine Tract Expansion Affecting the DYC Mutant of HOXD13, a New Murine Model of Synpolydactyly
Copyright Ó 2009 by the Genetics Society of America DOI: 10.1534/genetics.109.104695 Fork Stalling and Template Switching As a Mechanism for Polyalanine Tract Expansion Affecting the DYC Mutant of HOXD13, a New Murine Model of Synpolydactyly Olivier Cocquempot,*,†,1 Ve´ronique Brault,*,† Charles Babinet‡ and Yann Herault*,†,§,2 *Universite´ d’Orle´ans, UMR6218, Molecular Immunology and Embryology, 45071 Orle´ans, France †Centre National de la Recherche Scientifique (CNRS), UMR6218, MIE, 3B rue de la Fe´rollerie, 45071 Orleans Cedex 2, France, ‡Unite´ de Biologie du De´veloppement, URA 1960, CNRS, Institut Pasteur, 25 rue du Docteur Roux 75015 Paris, France and §CNRS, UPS44, TAAM, Institut de Transgenose, 45071 Orle´ans, France Manuscript received May 5, 2009 Accepted for publication June 15, 2009 ABSTRACT Polyalanine expansion diseases are proposed to result from unequal crossover of sister chromatids that increases the number of repeats. In this report we suggest an alternative mechanism we put forward while we investigated a new spontaneous mutant that we named ‘‘Dyc’’ for ‘‘Digit in Y and Carpe’’ phenotype. Phenotypic analysis revealed an abnormal limb patterning similar to that of the human inherited congenital disease synpolydactyly (SPD) and to the mouse mutant model Spdh. Both human SPD and mouse Spdh mutations affect the Hoxd13 gene within a 15-residue polyalanine-encoding repeat in the first exon of the gene, leading to a dominant negative HOXD13. Genetic analysis of the Dyc mutant revealed a trinucleotide expansion in the polyalanine-encoding region of the Hoxd13 gene resulting in a 7-alanine expansion. However, unlike the Spdh mutation, this expansion cannot result from a simple duplication of a short segment.