Modeling Congenital Disease and Inborn Errors of Development in Drosophila Melanogaster Matthew J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF File
Folia Morphol. Vol. 77, No. 2, pp. 386–392 DOI: 10.5603/FM.a2017.0080 O R I G I N A L A R T I C L E Copyright © 2018 Via Medica ISSN 0015–5659 www.fm.viamedica.pl Incidental imaging findings of congenital rib abnormalities: a case series and review of developmental concepts A.M. Aignătoaei1, C.E. Moldoveanu2, I.-D. Căruntu3, S.E. Giuşcă3, S. Partene Vicoleanu3, A.H. Nedelcu3 1Department of Pathology, “Sf. Spiridon” Clinic Hospital, 1, Independenţei Square, Iaşi, Romania 2Department of Radiology, Pneumology Clinic Hospital, 30, dr. Iosif Cihac Street, Iaşi, Romania 3Department of Morphofunctional Sciences, “Grigore T. Popa” University, 16, University Street, Iaşi, Romania [Received: 12 June 2017; Accepted: 24 July 2017] Background: Congenital rib abnormalities are found in approximately 2% of the general population. Usually, they occur in isolation and are rarely symptomatic, but they can also be associated with other malformations. Materials and methods: We reviewed imaging examinations performed over a period of 2 years (2014–2015), enabling us to identify isolated rib abnormalities in 6 adult patients. Results: The case series consisted in 3 cases with bilateral cervical ribs and 1 case each with bifid rib, costal fusion and rib pseudarthrosis. In all patients, the costal anomalies were discovered incidentally. All rib malformations were detected at thoracic radiography, except for the rib pseudarthrosis, which was identified at computed tomography (CT) scan. Differential diagnosis was made between cer- vical ribs and abnormalities of the C7 transverse process and of the first rib, while the other costal malformations were distinguished from tumoural, traumatic or inflammatory lesions of the chest wall, lung and pleura. -

Genetics of Congenital Hand Anomalies
G. C. Schwabe1 S. Mundlos2 Genetics of Congenital Hand Anomalies Die Genetik angeborener Handfehlbildungen Original Article Abstract Zusammenfassung Congenital limb malformations exhibit a wide spectrum of phe- Angeborene Handfehlbildungen sind durch ein breites Spektrum notypic manifestations and may occur as an isolated malforma- an phänotypischen Manifestationen gekennzeichnet. Sie treten tion and as part of a syndrome. They are individually rare, but als isolierte Malformation oder als Teil verschiedener Syndrome due to their overall frequency and severity they are of clinical auf. Die einzelnen Formen kongenitaler Handfehlbildungen sind relevance. In recent years, increasing knowledge of the molecu- selten, besitzen aber aufgrund ihrer Häufigkeit insgesamt und lar basis of embryonic development has significantly enhanced der hohen Belastung für Betroffene erhebliche klinische Rele- our understanding of congenital limb malformations. In addi- vanz. Die fortschreitende Erkenntnis über die molekularen Me- tion, genetic studies have revealed the molecular basis of an in- chanismen der Embryonalentwicklung haben in den letzten Jah- creasing number of conditions with primary or secondary limb ren wesentlich dazu beigetragen, die genetischen Ursachen kon- involvement. The molecular findings have led to a regrouping of genitaler Malformationen besser zu verstehen. Der hohe Grad an malformations in genetic terms. However, the establishment of phänotypischer Variabilität kongenitaler Handfehlbildungen er- precise genotype-phenotype correlations for limb malforma- schwert jedoch eine Etablierung präziser Genotyp-Phänotyp- tions is difficult due to the high degree of phenotypic variability. Korrelationen. In diesem Übersichtsartikel präsentieren wir das We present an overview of congenital limb malformations based Spektrum kongenitaler Malformationen, basierend auf einer ent- 85 on an anatomic and genetic concept reflecting recent molecular wicklungsbiologischen, anatomischen und genetischen Klassifi- and developmental insights. -

Orphanet Journal of Rare Diseases Biomed Central
Orphanet Journal of Rare Diseases BioMed Central Review Open Access Brachydactyly Samia A Temtamy* and Mona S Aglan Address: Department of Clinical Genetics, Human Genetics and Genome Research Division, National Research Centre (NRC), El-Buhouth St., Dokki, 12311, Cairo, Egypt Email: Samia A Temtamy* - [email protected]; Mona S Aglan - [email protected] * Corresponding author Published: 13 June 2008 Received: 4 April 2008 Accepted: 13 June 2008 Orphanet Journal of Rare Diseases 2008, 3:15 doi:10.1186/1750-1172-3-15 This article is available from: http://www.ojrd.com/content/3/1/15 © 2008 Temtamy and Aglan; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Brachydactyly ("short digits") is a general term that refers to disproportionately short fingers and toes, and forms part of the group of limb malformations characterized by bone dysostosis. The various types of isolated brachydactyly are rare, except for types A3 and D. Brachydactyly can occur either as an isolated malformation or as a part of a complex malformation syndrome. To date, many different forms of brachydactyly have been identified. Some forms also result in short stature. In isolated brachydactyly, subtle changes elsewhere may be present. Brachydactyly may also be accompanied by other hand malformations, such as syndactyly, polydactyly, reduction defects, or symphalangism. For the majority of isolated brachydactylies and some syndromic forms of brachydactyly, the causative gene defect has been identified. -

Medical Genetics and Genomic Medicine in the United States of America
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by George Washington University: Health Sciences Research Commons (HSRC) Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Pediatrics Faculty Publications Pediatrics 7-1-2017 Medical genetics and genomic medicine in the United States of America. Part 1: history, demographics, legislation, and burden of disease. Carlos R Ferreira George Washington University Debra S Regier George Washington University Donald W Hadley P Suzanne Hart Maximilian Muenke Follow this and additional works at: https://hsrc.himmelfarb.gwu.edu/smhs_peds_facpubs Part of the Genetics and Genomics Commons APA Citation Ferreira, C., Regier, D., Hadley, D., Hart, P., & Muenke, M. (2017). Medical genetics and genomic medicine in the United States of America. Part 1: history, demographics, legislation, and burden of disease.. Molecular Genetics and Genomic Medicine, 5 (4). http://dx.doi.org/10.1002/mgg3.318 This Journal Article is brought to you for free and open access by the Pediatrics at Health Sciences Research Commons. It has been accepted for inclusion in Pediatrics Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. GENETICS AND GENOMIC MEDICINE AROUND THE WORLD Medical genetics and genomic medicine in the United States of America. Part 1: history, demographics, legislation, and burden of disease Carlos R. Ferreira1,2 , Debra S. Regier2, Donald W. Hadley1, P. Suzanne Hart1 & Maximilian Muenke1 1National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland 2Rare Disease Institute, Children’s National Health System, Washington, District of Columbia Correspondence Carlos R. -

Part II Thoracic Spine
Open Access Review Article DOI: 10.7759/cureus.8684 Anatomical Variations That Can Lead to Spine Surgery at The Wrong Level: Part II Thoracic Spine Manan Shah 1 , Dia R. Halalmeh 2 , Aubin Sandio 1 , R. Shane Tubbs 3, 4, 5 , Marc D. Moisi 2 1. Neurosurgery, Wayne State University, Detroit Medical Center, Detroit, USA 2. Neurosurgery, Detroit Medical Center, Detroit, USA 3. Neurosurgery and Structural & Cellular Biology, Tulane University School of Medicine, New Orleans, USA 4. Anatomical Sciences, St. George's University, St. George's, GRD 5. Neurosurgery and Ochsner Neuroscience Institute, Ochsner Health System, New Orleans, USA Corresponding author: Dia R. Halalmeh, [email protected] Abstract Spine surgery at the wrong level is a detrimental ordeal for both surgeon and patient, and it falls under the wrong-site surgery sentinel events reporting system. While there are several methods designed to limit the incidence of these events, they continue to occur and can result in significant morbidity for the patient and malpractice lawsuits for the surgeon. In thoracic spine, numerous risk factors influence the development of this misadventure. These include anatomical variations such as transitional vertebrae, rib variants, hemivertebra, and block/fused vertebrae as well as patient characteristics, such as tumors, infections, previous thoracic spine surgery, obesity, and osteoporosis. An extensive literature search of the PubMed database up to 2019 was completed on each of the anatomical entities and their influence on developing thoracic spine surgery at the wrong level, taking into consideration patient’s individual factors. A reliable protocol and effective techniques were described to prevent this error. -
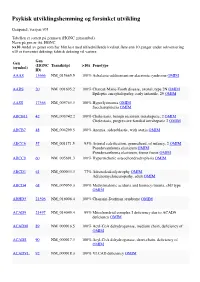
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Nova Scotia Atlee Perinatal Database Coding Manual 8Th Edition (Version 8.0)
Nova Scotia Atlee Perinatal Database Coding Manual 8th Edition (Version 8.0) April 2001 NOVA SCOTIA ATLEE PERINATAL DATABASE CODING MANUAL TABLE OF CONTENTS (A) ROUTINE INFORMATION LISTING OF HOSPITALS .......................1 DELIVERED ADMISSIONS ......................3 UNDELIVERED ADMISSIONS ..................59 POSTPARTUM ADMISSIONS ...................69 NEONATAL ADMISSIONS .................... 79 ANOMALY ADMISSIONS ......................91 ................................................. (B) CLASSIFICATION OF MATERNAL DISEASES AND PROCEDURES (Blue Section) I. Previous Pregnancy Maternal Diseases.............. 01 II. Present Pregnancy Maternal Diseases............... 02 A. Obstetrical ..............................02 B. Non-Obstetrical..........................07 C. Labour Complications .....................15 D. Non-Delivery Procedures ..................16 E. Analgesia During Labour ...................20 F. Anesthesia During Labour/Delivery ..........22 G. Anesthesia for Non-Delivery Procedures ......24 H. Lacerations ..............................25 I. Postpartum Complications..................26 J. Postpartum Infections .....................29 K. Maternal Therapy.........................31 L. Maternal Death or Undelivered Fetal Death....33 M. Infection in Present Pregnancy .............34 N. Complications of Anesthesia................37 O. Antibiotic Therapy........................39 P. Fetal Procedures..........................40 Q. Methods of Induction ......................41 8th Edition, April 2001, V8.0 Table of Contents and -

Table of Contents
Table of contents Acknowledgements I List of Figures III List of Tables XI List of Abbreviations XIV Summary XVI Part 1 Primary microcephaly 1 INTRODUCTION 1 1.1 Primary microcephaly (MCPH) 2 1.2 Structure of cerebral cortex 5 1.3 Development of cerebral cortex 7 1.4 Clinical features of primary microcephaly 8 1.5 MCPH genes and their function 10 1.5.1 Microcephalin (MCPH1) 10 1.5.2 Abnormal spindle like microcephaly associated (ASPM) 11 1.5.3 Cyclin dependent kinase5 regulatory associated protein (CDK5RAP2) 12 1.5.4 Centrosomal protein J (CENPJ) 13 1.5.5 STIL (SCL/TAL1 interrupting locus) 13 1.6 Objectives of study 16 2 MATERIALS AND METHODS 17 2.1 Families studied 17 2.1.1 MCP3 18 2.1. 2 MCP6 19 2.1.3 MCP7 20 2.1.4 MCP9 21 2.1.5 MCP11 22 2.1.6 MCP15 23 2.1. 7 MCP17 24 2.1.8 MCP18 25 2.1.9 MCP21 26 2.1.10 MCP22 27 2.1.11 MCP35 28 2.1.12 MCP36 29 2.2 Clinical data 29 2.3 Blood sampling 32 2.4 DNA extraction 33 2.5 Polymerase chain reaction 33 2.6 Agarose gel electrophoresis 33 2.7 Genotyping using fluorescently labeled primers 34 2.8 Preparation of samples for ABI 3130xl genetic analyzer 35 2.9 Linkage analysis 35 2.10 Mutation screening 36 2.10.1 DNA sequencing 36 2.10.2 Purification of PCR product from agarose gel 37 2.10.3 Purification of PCR product 37 2.10.4 Sequencing PCR reactions 37 2.10.5 Precipitation of sequencing PCR products 38 2.10.6 Sequencing data analysis 38 2.11 Restriction analysis 39 3 RESULTS 51 3.1 Linkage studies 51 3.2 Mutation analysis 52 3.2.1 Sequencing of ASPM in MCPH5 linked families 52 3.2.2 Compound heterozygous -

Jaw Keratocysts and Sotos Syndrome
International Journal on Oral Health Jaw Keratocysts and Sotos Syndrome Case Report Volume 1 Issue 2- 2021 Author Details Jin Fei Yeo and Philip McLoughlin* Faculty of Dentistry, National University Centre for Oral Health Singapore, Singapore *Corresponding author Philip McLoughlin, Discipline of Oral & Maxillofacial Surgery, Faculty of Dentistry, National University Centre for Oral Health Singapore, 9 Lower Kent Ridge Road, Singapore Article History Received: June 05, 2021 Accepted: June 14, 2021 Published: June 15, 2021 Abstract Sotos syndrome, described by Sotos et al. [1], is characterized by excessive growth during childhood, macrocephaly, distinctive facial appearance and learning disability. The disorder is largely caused by mutations or deletions in the NSD1 gene. The typical facial gestalt includes macrodolichocephaly with frontal bossing, front-parietal sparseness of hair, apparent hypertelorism, down slanting palpebral in his jaw bones, a previously unreported oral manifestation, out with a syndromic context. fissures, and facial flushing. This paper discusses a case of Sotos syndrome in an adolescent male with multiple odontogenic keratocysts Introduction On examination, he was a generally healthy and rather active patient. He was very tall with macrocephaly, large hands and feet, and Sotos syndrome (Cerebral gigantism) is a rare genetic childhood hypertelorism with slightly down-slanted eyes. He also suffered ASD/ overgrowth condition described in 1964 by Sotos et al. [1]. This autism and mild mental hypo development with noticeable speech syndrome is characterized by excessive growth during childhood, impairment. On clinical assessment he was found to have a number of macrocephaly, distinctive facial appearance and learning disability. unerupted teeth, with fluctuant expansion of the maxilla and mandible The disorder is caused by mutations or 5q35 microdeletions in the at those sites. -

Daniel Lopo Polla.Pdf
Pró-Reitoria de Pós-Graduação e Pesquisa Programa de Pós-Graduação Stricto Sensu em Ciências Genômicas e Biotecnologia SEQUENCIAMENTO DE EXOMA PARCIAL COMO FERRAMENTA DE DIAGNÓSTICO MOLECULAR DE DISPLASIAS ESQUELÉTICAS Autor: Daniel Lôpo Polla Orientador: Dr. Robert Pogue Coorientador: Dr. Rinaldo Wellerson Pereira Brasília - DF 2014 DANIEL LÔPO POLLA SEQUENCIAMENTO DE EXOMA PARCIAL COMO FERRAMENTA DE DIAGNÓSTICO MOLECULAR DE DISPLASIAS ESQUELÉTICAS Dissertação apresentada ao Programa de Pós-graduação Stricto Sensu em Ciências Genômicas e Biotecnologia da Universidade Católica de Brasília, como requisito parcial para a obtenção do Título de Mestre em Ciências Genômicas e Biotecnologia. Orientador: Dr. Robert Pogue Coorientador: Dr. Rinaldo Wellerson Pereira Brasília 2014 P771s Polla, Daniel Lôpo. Sequenciamento de exoma parcial como ferramenta de diagnóstico molecular de displasias esqueléticas. / Daniel Lôpo Polla – 2014. 133 f.; il : 30 cm Dissertação (mestrado) – Universidade Católica de Brasília, 2014. Orientação: Prof. Dr. Robert Pogue Coorientação: Prof. Dr. Rinaldo Wellerson Pereira 1. Biotecnologia. 2. Diagnóstico molecular. 3. Displasia esquelética. I. Pogue, Robert, orient. II. Pereira, Rinaldo Wllerson, coorient. III. Título. CDU 577.21 Ficha elaborada pela Biblioteca Pós-Graduação da UCB Dissertação de autoria de Daniel Lôpo Polla, intitulada “SEQUENCIAMENTO DE EXOMA PARCIAL COMO FERRAMENTA DE DIAGNÓSTICO MOLECULAR DE DISPLASIAS ESQUELÉTICAS”, apresentada como requisito parcial para a obtenção de grau de Mestre em Ciências Genômicas e Biotecnologia da Universidade Católica de Brasília, em 12 de março de 2014, defendida e aprovada pela banca examinadora abaixo assinada: Brasília 2014 AGRADECIMENTOS Agradeço em primeiro lugar a Deus que iluminou o meu caminho durante mais esta caminhada, dando-me força e paciência para superar os desafios. -

Medical Genetics and Genomic Medicine in the United States of America
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Pediatrics Faculty Publications Pediatrics 7-1-2017 Medical genetics and genomic medicine in the United States of America. Part 1: history, demographics, legislation, and burden of disease. Carlos R Ferreira George Washington University Debra S Regier George Washington University Donald W Hadley P Suzanne Hart Maximilian Muenke Follow this and additional works at: https://hsrc.himmelfarb.gwu.edu/smhs_peds_facpubs Part of the Genetics and Genomics Commons APA Citation Ferreira, C., Regier, D., Hadley, D., Hart, P., & Muenke, M. (2017). Medical genetics and genomic medicine in the United States of America. Part 1: history, demographics, legislation, and burden of disease.. Molecular Genetics and Genomic Medicine, 5 (4). http://dx.doi.org/10.1002/mgg3.318 This Journal Article is brought to you for free and open access by the Pediatrics at Health Sciences Research Commons. It has been accepted for inclusion in Pediatrics Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. GENETICS AND GENOMIC MEDICINE AROUND THE WORLD Medical genetics and genomic medicine in the United States of America. Part 1: history, demographics, legislation, and burden of disease Carlos R. Ferreira1,2 , Debra S. Regier2, Donald W. Hadley1, P. Suzanne Hart1 & Maximilian Muenke1 1National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland 2Rare Disease Institute, Children’s National Health System, Washington, District of Columbia Correspondence Carlos R. Ferreira, National Human Genome Research Institute, National Institutes of Health, 10 Center Drive, Building 10, Room 10C103, Bethesda, Maryland 20892-1851. -

PTCH1 Duplication in a Family with Microcephaly and Mild Developmental Delay
European Journal of Human Genetics (2009) 17, 267 – 271 & 2009 Macmillan Publishers Limited All rights reserved 1018-4813/09 $32.00 www.nature.com/ejhg SHORT REPORT PTCH1 duplication in a family with microcephaly and mild developmental delay Katarzyna Derwin´ ska1,2, Marta Smyk1,2, Mitchell Lance Cooper1, Patricia Bader3, Sau Wai Cheung1 and Paweł Stankiewicz*,1 1Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX, USA; 2Department of Medical Genetics, Institute of Mother and Child, Warsaw, Poland; 3Department of Cytogenetics, Parkview Memorial Hospital, Fort Wayne, IN, USA With the exception of the X chromosome, genomic deletions appear to be more prevalent than duplications. Because of a lack of accurate diagnostic methods, submicroscopic duplications have been under-ascertained for a long period. The development of array CGH has enabled the detection of chromosomal microduplications with nearly the same sensitivity as deletions, leading to the discovery of previously unrecognized syndromes. Using a clinical targeted oligonucleotide array (CMA-V6.3 OLIGO), we identified an B360-kb duplication in 9q22.32 in a 21-month-old boy with developmental delay, failure to thrive, and microcephaly. The same duplication was identified in the patient’s mother who is also microcephalic and mildly delayed. We have sequenced the chromosomal breakpoints and determined the duplication as tandem in orientation and 363 599 bp in size. The duplicated segment harbors the entire PTCH1 gene. Deletions or loss-of-function mutations of PTCH1 result in basal cell nevus syndrome (Gorlin syndrome), whereas gain-of-function mutations were proposed to lead to holoprosencephaly 7. We propose that patients with microcephaly or holoprosencephaly of unknown origin should also be screened for PTCH1 duplication.