Mft•] ~;;I~ [I) I~ T?L3 ·Ilr!F·S; [,J ~ M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-Tailed Opossum (Monodelphis Domestica) and North American Opossum (Didelphis Virginiana)
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2001 Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana) Lee Anne Cope University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Animal Sciences Commons Recommended Citation Cope, Lee Anne, "Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana). " PhD diss., University of Tennessee, 2001. https://trace.tennessee.edu/utk_graddiss/2046 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Lee Anne Cope entitled "Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana)." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Animal Science. Robert W. Henry, Major Professor We have read this dissertation and recommend its acceptance: Dr. R.B. Reed, Dr. C. Mendis-Handagama, Dr. J. Schumacher, Dr. S.E. Orosz Accepted for the Council: Carolyn R. -

Variation of Cystic Duct Insertion in Relation to the Extrahepatic Ducts
AbeshaAmbaye et al. / International Journal of Pharma Sciences and Research (IJPSR) Variation of Cystic Duct Insertion in Relation to the Extrahepatic Ducts and Observed Frequency of Double Lumen Apparent Common Bile Duct AbeshaAmbaye1, MueezAbraha2,Bernard A. Anderson2, Amanuel T. Tsegay1 Anatomy Course and Research Team, Institute of Biomedical Sciences, College of Health Sciences, Mekelle University Dep’t of Anatomy, College of Medicine and Health Sciences, University of Gondar, Ethiopia email [email protected] ABSTRACT Background: Variations in the pattern of the extra hepatic biliary tract are common and usually encountered during radiological investigations or during operations on the biliary tree. Having a good knowledge of the possible connections of the cystic duct with the common hepatic duct to form the common bile duct is very important; because variation in this area is common. Objectives: The main aim of this study is to evaluate the frequency of anatomic variations of the cystic duct insertion in relation to the extrahepatic ducts and Observed Frequency of Double Lumen Apparent Common Bile Duct Methods: Institutional based cross-sectional study design with observational data collection tool was conducted in 25 Ethiopian fixed cadavers and Forensic autopsy specimens obtained from Departments of Human Anatomy at University of Gondar, Mekelle and St. Paul Hospital Millennium Medical College Result: From the total 25 specimens dissected 9 (36%) had the ACBD and the 16 (64%) of them had CBD with one lumen. Conclusion: The billiary system formation is very variable, among the variants; the number of the supradoudenal insertion is greater than the infradoudenal insertion. ACBD is more frequent than expected which is 36% of the total data. -

Sphincter of Oddi: ERCP Plus Sphincterotomy – Yes Or No
Sphincter of Oddi: ERCP Plus Sphincterotomy – Yes or No Note: For debate purposes, the pro and con positions for patient management will be taken by the invited authors. However, actual decisions regarding patient care must involve discussion of the risks and benefits of each treatment considered. Case Presentation – Case developed by Ihab I. El Hajj, MD, MPH, Indiana University, Indianapolis, IN A 57-year-old Caucasian female with history of smoking and COPD, was in her usual state of health until two years ago, when she experienced recurrent “attacks” of right upper quadrant pain, nausea and occasional vomiting, suggestive of biliary colic. The patient was evaluated by her primary care physician and initial work- up, which included basic blood work, liver chemistries and transabdominal ultrasound, were negative. The patient responded partially to prn Zofran and omeprazole 40 mg once then twice daily. With the persistence of her symptoms, the patient was referred to a gastroenterologist. Esophagogastroduodenoscopy (EGD) with gastric biopsies revealed chronic inactive gastritis without Helicobacter pylori. HIDA scan suggested biliary dyskinesia with an ejection fraction of 22%. An elective laparoscopic cholecystectomy was performed. An intra- operative cholangiogram showed no filling defect in the common bile duct (CBD) and pathology demonstrated chronic cholecystitis with no gallstones. The patient was symptom-free for six months after surgery. She subsequently developed vague upper abdominal pain, intermittent nausea and irregular bowel movements. Labs, colonoscopy and repeat EGD were ASGE Leading Edge — Volume 4, No. 4 © American Society for Gastrointestinal Endoscopy normal. The patient was treated for suspected irritable bowel syndrome. She failed several medications including hyoscyamine, dicyclomine, amitriptyline, sucralfate, and GI cocktail. -
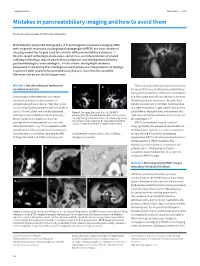
Mistakes in Pancreatobiliary Imaging and How to Avoid Them
ueg education Mistakes in… 2020 Mistakes in pancreatobiliary imaging and how to avoid them Marianna Arvanitakis and Martina Pezzullo Multidetector computed tomography (CT) and magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP) are cross-sectional imaging modalities largely used for patients with pancreatobiliary diseases.1–3 Despite recent technological advances, correct use and interpretation of related radiological findings require good clinical judgment and collaboration between gastroenterologists and radiologists. In this article, we highlight mistakes frequently made during the radiological investigation and interpretation of findings in patients with suspected pancreatobiliary diseases, based on the available literature and on our clinical experience. There are several biliary anatomic variations to Mistake 1 Not describing or looking for a b anatomical variants be aware of that may lead to perioperative biliary injury: perihilar insertion of the cystic duct defined Laparoscopic cholecystectomy is currently as a short cystic duct with an insertion <1 cm from the standard procedure for treatment of the hilum; posterior insertion of the cystic duct symptomatic gallstone disease.4 Bile duct injury into the common bile duct (CBD); direct insertion can occur during the procedure with an incidence of a segmental/sectoral right hepatic duct into the up to 0.7% and, albeit rare, can be associated Figure 1 | Imaging the cystic duct. a | 2D MRCP gallbladder or the cystic duct; and insertion of a with significant morbidity and even mortality.4 showing that what looks like the cystic duct (arrow) is right sectoral/segmental hepatic duct directly into Biliary anatomical variations can lead to actually the right posterior duct separately originating the CBD (figure 1).3,5 from the common bile duct. -

Suppression of Hepatocyte CYP1A2 Expression by Kupffer Cells Via Ahr Pathway: the Central Role of Proinflammatory Cytokines
339-346 29/6/06 12:40 Page 339 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 18: 339-346, 2006 339 Suppression of hepatocyte CYP1A2 expression by Kupffer cells via AhR pathway: The central role of proinflammatory cytokines RONGQIAN WU1, XIAOXUAN CUI1, WEIFENG DONG1, MIAN ZHOU1, H. HANK SIMMS2 and PING WANG1 1Department of Surgery, North Shore University Hospital and Long Island Jewish Medical Center, Manhasset, NY 11030, USA Received February 6, 2006; Accepted March 23, 2006 Abstract. The hepatic cytochrome P-450 (CYP) enzyme such downregulation. Inhibition of proinflammatory cytokines system provides a major aspect of liver function, yet alterations by curcumin may provide a novel approach to modulate the of CYP in sepsis remain largely unknown. Although we have hepatic CYP function in sepsis. recently shown that CYP1A2, one of the major isoforms of CYP in rats, is downregulated in sepsis, the underlying mech- Introduction anism and possible therapeutic approaches warrant further investigation. The aim of this study was to determine whether Sepsis is the leading cause of death in non-cardiac intensive Kupffer cells (KCs) play any role in suppressing CYP1A2 in care units with >210,000 people succumbing to overwhelming the hepatocytes (HCs) and if so, how to modulate CYP1A2 infection (or the resultant multiple organ failure) in the US expression in sepsis. To study this, primary KCs and HCs annually (1). Although experimental studies using cell and were cultured separately or together with or without transwells. animal models have greatly improved our understanding of Cells and supernatant samples were collected after various the pathophysiology of sepsis, there remains a remarkable stimulations. -

Hepatocyte Growth Factor Signaling Pathway As a Potential Target in Ductal Adenocarcinoma of the Pancreas
JOP. J Pancreas (Online) 2017 Nov 30; 18(6):448-457. REVIEW ARTICLE Hepatocyte Growth Factor Signaling Pathway as a Potential Target in Ductal Adenocarcinoma of the Pancreas Samra Gafarli, Ming Tian, Felix Rückert Department of Surgery, Medical Faculty Mannheim, University of Heidelberg, Germany ABSTRACT Hepatocyte growth factor is an important cellular signal pathway. The pathway regulates mitogenesis, morphogenesis, cell migration, invasiveness and survival. Hepatocyte growth factor acts through activation of tyrosine kinase receptor c-Met (mesenchymal epithelial transition factor) as the only known ligand. Despite the fact that hepatocyte growth factor is secreted only by mesenchymal origin cells, the targets of this multifunctional pathway are cells of mesenchymal as well as epithelial origin. Besides its physiological role recent evidences suggest that HGF/c-Met also plays a role in tumor pathophysiology. As a “scatter factor” hepatocyte growth factor stimulates cancer cell migration, invasion and subsequently promote metastases. Hepatocyte growth factor further is involved in desmoplastic reaction and consequently indorse chemo- and radiotherapy resistance. Explicitly, this pathway seems to mediate cancer cell aggressiveness and to correlate with poor prognosis and survival rate. Pancreatic Ductal Adenocarcinoma is a carcinoma with high aggressiveness and metastases rate. Latest insights show that the HGF/c-Met signal pathway might play an important role in pancreatic ductal adenocarcinoma pathophysiology. In the present review, we highlight the role of HGF/c-Met pathway in pancreatic ductal adenocarcinoma with focus on its effect on cellular pathophysiology and discuss its role as a potential therapeutic target in pancreatic ductal adenocarcinoma. INTRODUCTION activation causes auto-phosphorylation of c-Met and subsequent activation of downstream signaling pathways Hepatocyte growth factor (HGF) is a multifunctional such as mitogen-activated protein kinases (MAPKs), gene. -

Hepatic Toxicity
Hepatic Toxicity MSc in Molecular Pathology and Toxicology 2001 Andy Smith MRC Toxicology Unit THE LIVER • The liver constitutes about 5% of the body mass of a rodent or human. • It has many functions eg. Carbohydrate storage and metabolism Synthesis of fibrinogen and albumin etc. Fat metabolism Synthesis of bile acids Metabolism of hormones Formation of urea from amino acids • Blood supply is about 20% arterial-80% venous. • May contain 10-15% of blood volume INFLUENCE OF TOXIC CHEMICALS ON THE LIVER • The liver is the most common site of damage in laboratory animals administered drugs and other chemicals. There are many reasons including the fact that the liver is the first major organ to be exposed to ingested chemicals due to its portal blood supply. • Although chemicals are delivered to the liver to be metabolized and excreted, this can frequently lead to activation and liver injury. • Study of the liver has been and continues to be important in understanding fundamental molecular mechanisms of toxicity as well as in assessment of risks to humans. VENA CAVA HEPATIC VEIN LIVER HEPATIC PORTAL VEIN BILE DUCT SMALL AND LARGE INTESTINE LOBULE PORTAL VEIN BLOOD FLOW HEPATIC ARTERY III I BILE FLOW BILE DUCT CENTRAL VEIN PORTAL TRIAD TYPES OF LIVER CELLS Hepatocytes- Not all the same; depends on lobular site Zone I Higher in respiratory enzymes (periportal) Zone III Higher in cytochrome P450 (centrilobular) Endothelial cells Bile duct cells Oval cells- Possibly stem cells Kupffer cells- Phagocytic cells Important role in inflammation Ito cells- Fat storing or stellate cells TYPES OF HEPATIC INJURY OR RESPONSES Each of the different cell types may respond to a toxic insult. -

Alcohol and Hepatocyte-Kupffer Cell Interaction (Review)
MOLECULAR MEDICINE REPORTS 4: 597-602, 2011 Alcohol and hepatocyte-Kupffer cell interaction (Review) MICHAEL AJAKAIYE, ASHA JACOB, RONGQIAN WU, JEFFREY M. NICASTRO, GENE F. COPPA and PING WANG Department of Surgery, North Shore University Hospital-Long Island Jewish Medical Center, and Laboratory of Surgical Research, The Feinstein Institute for Medical Research, Manhasset, NY, USA Received December 15, 2010; Accepted March 23, 2011 DOI: 10.3892/mmr.2011.471 Abstract. Alcoholic liver disease accounts for 12,000 deaths 1. Introduction per year in the United States and is the second leading indica- tion for liver transplantation. It covers a spectrum of disease … if the surfeit of delicacies, or the hereditary wine conditions ranging from steatosis and cirrhosis to hepatic of my country dared to disturb my health or the malignancies. Epidemiological data clearly show a strong equilibrium of my poetry, from you, dark monarch, correlation between alcohol consumption and liver diseases. giver of syrups and of poisons, regulator of salts, A large body of evidence has accumulated over the years in from you I hope for justice: I love life: Do not betray determining the molecular mediators of alcohol-induced liver me! Work on! injury. In this review, we provide an overview of such media- —Pablo Neruda, Oda al Higado (1) tors, which include alcohol metabolites and reactive oxygen/ nitrogen species, endotoxin via bacterial translocation from Alcoholic liver disease (ALD) affects 1% of the North the gut and TNF-α, and highlight the role of the sympathetic American population and accounted for over 12,000 deaths in nervous stimuli, norepinephrine and the α2A-adrenergic 2001. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Anatomy of the Digestive System
The Digestive System Anatomy of the Digestive System We need food for cellular utilization: organs of digestive system form essentially a long !nutrients as building blocks for synthesis continuous tube open at both ends !sugars, etc to break down for energy ! alimentary canal (gastrointestinal tract) most food that we eat cannot be directly used by the mouth!pharynx!esophagus!stomach! body small intestine!large intestine !too large and complex to be absorbed attached to this tube are assorted accessory organs and structures that aid in the digestive processes !chemical composition must be modified to be useable by cells salivary glands teeth digestive system functions to altered the chemical and liver physical composition of food so that it can be gall bladder absorbed and used by the body; ie pancreas mesenteries Functions of Digestive System: The GI tract (digestive system) is located mainly in 1. physical and chemical digestion abdominopelvic cavity 2. absorption surrounded by serous membrane = visceral peritoneum 3. collect & eliminate nonuseable components of food this serous membrane is continuous with parietal peritoneum and extends between digestive organs as mesenteries ! hold organs in place, prevent tangling Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 1 Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 2 is suspended from rear of soft palate The wall of the alimentary canal consists of 4 layers: blocks nasal passages when swallowing outer serosa: tongue visceral peritoneum, -

Surgical Significance of Variations in Anatomy in the Biliary Region
International Journal of Research in Medical Sciences Hassan AU et al. Int J Res Med Sci. 2013 Aug;1(3):xx-xx www.msjonline.org pISSN 2320-6071 | eISSN 2320-6012 DOI: 10.5455/2320-6012.ijrms20130812 Review Article Surgical significance of variations in anatomy in the biliary region Ashfaq Ul Hassan1*, Showqat A. Zargar2, Aijaz Malik3, Pervez Shah4 1Department of Anatomy, SKIMS Medical College, Srinagar, Kashmir, India 2Department of Gastroenterology, SKIMS, Srinagar, Kashmir, India 3Department of Surgery, SKIMS, Srinagar, Kashmir, India 4Department of Medicine, SMHS Hospital, Srinagar, Kashmir, India Received: 8 May 2013 Accepted: 21 May 2013 *Correspondence: Dr. Ashfaq Ul Hassan, E-mail: [email protected] © 2013 Hassan AU et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Variations in the anatomy of the gallbladder, the bile ducts, and the arteries that supply them and the liver are important to the surgeon, because failure to recognize them can cause iatrogenic injury to the biliary tract. A surgeon should be always be careful while operating in this area. In addition these anomalies are associated with a range of other congenital anomalies, including biliary atresia and cardiovascular or other gastrointestinal malformations, biliary lithiasis, choledochal cyst, anomalous pancreaticobiliary junction etc, so a look out for other anomalies should be carried out simultaneously. Keywords: Gall bladder, Cystic fossa, Calots triangle, Monyhans hump, Extrahepatic, Atresia, Double Gall bladder, Triple Gall bladder, Agenesis, Cystic duct, Carolis disease NORMAL ANATOMY the left from the neck of the gallbladder, and joins the common hepatic duct to form the common bile duct. -

Cystic Duct Cholangiography Leo Chaikof
Henry Ford Hospital Medical Journal Volume 22 Number 3 Laurence S. Fallis, M.D. Commemorative Article 7 Issue 9-1974 Cystic Duct Cholangiography Leo Chaikof T. L. Friedlich R. A. Affifi H. Weizel Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Chaikof, Leo; Friedlich, T. L.; Affifi, R. A.; and Weizel, H. (1974) "Cystic Duct Cholangiography," Henry Ford Hospital Medical Journal : Vol. 22 : No. 3 , 129-136. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol22/iss3/7 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp. Med. Journal Vol. 22, No. 3, 1974 Cystic Duct Cholangiography Leo Chaikof, MD,* T.L. Friedlich, MD, R.A. Affifi, MD and H. Weizel, MD ALTHOUGH operative cholangiog raphy was first used in 1932 by Mirizzi,^'^ it is still not done routinely as part of the surgical procedure in biliary tract opera tions. According to Jolly, Baker et al,^ only 18% of members of the American Surgical Association use it routinely. De spite a great deal of discussion pro and con in the literature,^'" it appears that, if A series of 837 cystic duct cholangiograms the frequency of common duct explora has been reviewed. The technique is simple and safe to carry out. It is not time consuming tion can be reduced, certainly its as and does not require any unusual equipment.