Primary Sclerosing Cholangitis: Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inflammatory Bowel Disease Irritable Bowel Syndrome
Inflammatory Bowel Disease and Irritable Bowel Syndrome Similarities and Differences 2 www.ccfa.org IBD Help Center: 888.MY.GUT.PAIN 888.694.8872 Important Differences Between IBD and IBS Many diseases and conditions can affect the gastrointestinal (GI) tract, which is part of the digestive system and includes the esophagus, stomach, small intestine and large intestine. These diseases and conditions include inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). IBD Help Center: 888.MY.GUT.PAIN 888.694.8872 www.ccfa.org 3 Inflammatory bowel diseases are a group of inflammatory conditions in which the body’s own immune system attacks parts of the digestive system. Inflammatory Bowel Disease Inflammatory bowel diseases are a group of inflamma- Causes tory conditions in which the body’s own immune system attacks parts of the digestive system. The two most com- The exact cause of IBD remains unknown. Researchers mon inflammatory bowel diseases are Crohn’s disease believe that a combination of four factors lead to IBD: a (CD) and ulcerative colitis (UC). IBD affects as many as 1.4 genetic component, an environmental trigger, an imbal- million Americans, most of whom are diagnosed before ance of intestinal bacteria and an inappropriate reaction age 35. There is no cure for IBD but there are treatments to from the immune system. Immune cells normally protect reduce and control the symptoms of the disease. the body from infection, but in people with IBD, the immune system mistakes harmless substances in the CD and UC cause chronic inflammation of the GI tract. CD intestine for foreign substances and launches an attack, can affect any part of the GI tract, but frequently affects the resulting in inflammation. -

Nutritional Considerations in Inflammatory Bowel Disease
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #5 Series Editor: Carol Rees Parrish, M.S., R.D., CNSD Nutritional Considerations in Inflammatory Bowel Disease by Kelly Anne Eiden, M.S., R.D., CNSD Nutrient alterations are commonplace in patients with inflammatory bowel disease. The etiology for these alterations is multifactorial. Nutrition assessment is the first step in successful nutrition management of any patient with gastrointestinal disease. Nutritional goals include assisting with nutrition risk, identifying macronutrient and micronutrient needs and implementing a nutrition plan to meet those needs. This article addresses many of the nutrition issues currently facing clinicians including: oral, enteral and parenteral nutrition, common vitamin/mineral deficiencies, medium chain triglycerides and nutrition as primary and supportive therapy. INTRODUCTION and supportive treatment in both Crohn’s and UC. The nflammatory bowel disease (IBD), encompassing following article will provide guidelines to help the both Crohn’s disease and ulcerative colitis (UC), is clinician determine nutritional risk, review specialized Ia chronic inflammatory intestinal disorder of nutrient needs and discuss nutrition as a treatment unknown etiology. A multitude of factors, including modality in the patient with IBD. drug-nutrient interactions, disease location, symp- toms, and dietary restrictions can lead to protein NUTRITION ASSESSMENT IN INFLAMMATORY energy malnutrition and specific nutritional deficien- BOWEL DISEASE cies. It is estimated that up to 85% of hospitalized IBD patients have protein energy malnutrition, based on Factors Affecting Nutritional Status abnormal anthropometric and biochemical parameters in the Patient with IBD (1,2). As Crohn’s disease can occur anywhere from There are many factors that alter nutrient intake in the mouth to anus (80% of cases in the terminal ileum), it patient with IBD. -

Papilla with Separate Bile and Pancreatic Duct Orifices
JOP. J Pancreas (Online) 2013 May 10; 14(3):302-303. MULTIMEDIA ARTICLE – Clinical Imaging Papilla with Separate Bile and Pancreatic Duct Orifices Surinder Singh Rana, Deepak Kumar Bhasin Department of Gastroenterology, Post Graduate Institute of Medical Education and Research (PGIMER). Chandigarh, India A 32-year-old male, a known case of alcohol related Conflict of interest The authors have no potential chronic non calcific pancreatitis, was referred to us for conflicts of interest pancreatic endotherapy for relief of intractable abdominal pain. The cross sectional imaging studies References had revealed an irregularly dilated main pancreatic duct. The examination of the major duodenal papilla 1. Silvis SE, Vennes JA, Dreyer M. Variation in the normal duodenal papilla. Gastrointest Endosc 1983; 29:132-133 [PMID; revealed the presence of two separate orifices at 6852473] endoscopic retrograde cholangiopancreatography (ERCP) (Image). The cranial orifice was located at 11- 12 clock position whereas the caudal orifice was located at 4-5 clock position. The caudal orifice was selectively cannulated and the injection of the contrast revealed presence of an irregularly dilated main pancreatic duct. The cannula and the guide wire introduced through the caudal orifice selectively entered the pancreatic duct and did not come out through the cranial orifice. During ERCP, bile could be seen coming out of the cranial orifice, confirming it to be the orifice of common bile duct. Following selective cannulation of the main pancreatic duct, a 5-Fr stent was placed into the pancreatic duct. Following this, the patient had complete pain relief and is planned for further sessions of pancreatic endotherapy along with pancreatic sphincterotomy. -

Variation of Cystic Duct Insertion in Relation to the Extrahepatic Ducts
AbeshaAmbaye et al. / International Journal of Pharma Sciences and Research (IJPSR) Variation of Cystic Duct Insertion in Relation to the Extrahepatic Ducts and Observed Frequency of Double Lumen Apparent Common Bile Duct AbeshaAmbaye1, MueezAbraha2,Bernard A. Anderson2, Amanuel T. Tsegay1 Anatomy Course and Research Team, Institute of Biomedical Sciences, College of Health Sciences, Mekelle University Dep’t of Anatomy, College of Medicine and Health Sciences, University of Gondar, Ethiopia email [email protected] ABSTRACT Background: Variations in the pattern of the extra hepatic biliary tract are common and usually encountered during radiological investigations or during operations on the biliary tree. Having a good knowledge of the possible connections of the cystic duct with the common hepatic duct to form the common bile duct is very important; because variation in this area is common. Objectives: The main aim of this study is to evaluate the frequency of anatomic variations of the cystic duct insertion in relation to the extrahepatic ducts and Observed Frequency of Double Lumen Apparent Common Bile Duct Methods: Institutional based cross-sectional study design with observational data collection tool was conducted in 25 Ethiopian fixed cadavers and Forensic autopsy specimens obtained from Departments of Human Anatomy at University of Gondar, Mekelle and St. Paul Hospital Millennium Medical College Result: From the total 25 specimens dissected 9 (36%) had the ACBD and the 16 (64%) of them had CBD with one lumen. Conclusion: The billiary system formation is very variable, among the variants; the number of the supradoudenal insertion is greater than the infradoudenal insertion. ACBD is more frequent than expected which is 36% of the total data. -

Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease
pathogens Article Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease Patients from Southern Italy: A Case-Control Study Giuseppe Losurdo 1,2 , Andrea Iannone 1, Antonella Contaldo 1, Michele Barone 1 , Enzo Ierardi 1 , Alfredo Di Leo 1,* and Mariabeatrice Principi 1 1 Section of Gastroenterology, Department of Emergency and Organ Transplantation, University “Aldo Moro” of Bari, 70124 Bari, Italy; [email protected] (G.L.); [email protected] (A.I.); [email protected] (A.C.); [email protected] (M.B.); [email protected] (E.I.); [email protected] (M.P.) 2 Ph.D. Course in Organs and Tissues Transplantation and Cellular Therapies, Department of Emergency and Organ Transplantation, University “Aldo Moro” of Bari, 70124 Bari, Italy * Correspondence: [email protected]; Tel.: +39-080-559-2925 Received: 14 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: We performed an epidemiologic study to assess the prevalence of chronic viral hepatitis in inflammatory bowel disease (IBD) and to detect their possible relationships. Methods: It was a single centre cohort cross-sectional study, during October 2016 and October 2017. Consecutive IBD adult patients and a control group of non-IBD subjects were recruited. All patients underwent laboratory investigations to detect chronic hepatitis B (HBV) and C (HCV) infection. Parameters of liver function, elastography and IBD features were collected. Univariate analysis was performed by Student’s t or chi-square test. Multivariate analysis was performed by binomial logistic regression and odds ratios (ORs) were calculated. We enrolled 807 IBD patients and 189 controls. Thirty-five (4.3%) had chronic viral hepatitis: 28 HCV (3.4%, versus 5.3% in controls, p = 0.24) and 7 HBV (0.9% versus 0.5% in controls, p = 0.64). -

Fact Sheet - Symptoms of Pancreatic Cancer
Fact Sheet - Symptoms of Pancreatic Cancer Diagnosis Pancreatic cancer is often difficult to diagnose, because the pancreas lies deep in the abdomen, behind the stomach, so tumors are not felt during a physical exam. Pancreatic cancer is often called the “silent” cancer because the tumor can grow for many years before it causes pressure, pain, or other signs of illness. When symptoms do appear, they can vary depending on the size of the tumor and where it is located on the pancreas. For these reasons, the symptoms of pancreatic cancer are seldom recognized until the cancer has progressed to an advanced stage and often spread to other areas of the body. General Symptoms Pain The first symptom of pancreatic cancer is often pain, because the tumors invade nerve clusters. Pain can be felt in the stomach area and/or in the back. The pain is generally worse after eating and when lying down, and is sometimes relieved by bending forward. Pain is more common in cancers of the body and tail of the pancreas. The abdomen may also be generally tender or painful if the liver, pancreas or gall bladder are inflamed or enlarged. It is important to keep in mind that there are many other causes of abdominal and back pain! Jaundice More than half of pancreatic cancer sufferers have jaundice, a yellowing of the skin and whites of the eyes. Jaundice is caused by a build-up bilirubin, a substance which is made in the liver and a component of bile. Bilirubin contains a lot of yellow pigment, and gives bile it’s color. -

Common Bile Duct Exploration
Education Common Bile Duct Exploration What is a common bile duct exploration? The common bile duct is a tube that connects the liver, gallbladder, and pancreas to the small intestine. It helps deliver fluids for digestion. A common bile duct exploration is a procedure used to see if a stone is blocking the flow of bile from your liver and gallbladder to your intestine. When is it used? When a stone gets stuck in the common bile duct it may cause bile to back up into the liver. This causes jaundice. Jaundice is a condition in which the skin and the whites of the eyes become yellowish. If the stone is not removed, the common bile duct may become infected and need emergency surgery. It can also cause pancreatitis, a reaction in the pancreas that can be life threatening. Common bile duct exploration is often done during surgery to remove the gallbladder. An alternative procedure is an endoscopic retrograde cholangiopancreatography (ERCP). When an ERCP is done, a tube is inserted through your mouth and stomach into the small intestine. The tube can be used to put contrast dye into the duct to look for stones with x-rays. If there are stones, a small opening is made in the common duct to allow the stone or stones to pass into the intestine. You should ask your health care provider about these choices. How do I prepare for a common bile duct exploration? Plan for your care and recovery after the operation. Allow for time to rest and try to find people to help you with your day-to- day duties. -
![Mft•] ~;;I~ [I) I~ T?L3 ·Ilr!F·S; [,J ~ M](https://docslib.b-cdn.net/cover/6471/mft-i-i-i-t-l3-%C2%B7ilr-f%C2%B7s-j-m-706471.webp)
Mft•] ~;;I~ [I) I~ T?L3 ·Ilr!F·S; [,J ~ M
Mft•] ~;;I~ [I) I~ t?l3 ·ilr!f·S; [,j ~ M Hepatobiliary Imaging Update Maggie Chester and Jerry Glowniak Veterans Affairs Medical Center and Oregon Health Sciences University, Portland, Oregon and the gallbladder ejection fraction (EF) after the injection This is the first article in a four-part series on interventional of cholecystokinin (CCK) (Kinevac®, Squibb Diagnostics, nuclear medicine. Upon completion, the nuclear medicine New Brunswick, NJ). A brief description of the hepatic ex technologist should be able to (1) list the advantages of using traction fraction (HEF) was given; the technique used quan interventional hepatic imaging, (2) identify the benefit in tifies hepatocyte function more accurately than does excretion calculating HEF, and (3) utilize the HEF calculation method when appropriate. half-time. Since publication of the previous article (5), the HEF has become more widely used as a measure of hepatocyte function, and nearly all the major nuclear medicine software vendors include programs for calculating the HEF. Scintigraphic assessment of hepatobiliary function began in In this article, we will describe new observations and meth the 1950s with the introduction of iodine-131 C31 1) Rose ods used in hepatobiliary imaging. The following topics will bengal (1). Due to the poor imaging characteristics of 1311, be discussed: ( 1) the use of morphine as an aid in the diagnosis numerous attempts were made to find a technetium-99m 99 of acute cholecystitis, (2) the rim sign in the diagnosis of acute ( mTc) labeled hepatobiliary agent (2). The most useful of cholecystitis, and (3) methods for calculating the HEF. the several 99mTc-labeled agents that were investigated were the iminodiacetic acid (IDA) analogs, which were introduced MORPHINE-AUGMENTED CHOLESCINTIGRAPHY in the mid 1970s (3). -

Liver Disease in Ulcerative Colitis: an Epidemiological and Follow up Study
84 Gut 1994; 35:84-89 Liver disease in ulcerative colitis: an epidemiological and follow up study in the county of Stockholm Gut: first published as 10.1136/gut.35.1.84 on 1 January 1994. Downloaded from U Broome, H Glaumann, G Hellers, B Nilsson, J Sorstad, R Hultcrantz Abstract sclerosing cholangitis (PSC) are said to be more In an epidemiological study ofthe incidence of common in patients with UC.47 The incidence of ulcerative colitis (UC) in the county of Stock- these complications varies in different studies holm between 1955 and 1979, 1274 patients depending on selection criteria and on the with UC were discovered. Almost all these definition of hepatobiliary disease.89 The true patients had regularly been investigated with incidence of hepatobiliary dysfunction in UC, liver function tests; 142 (11%) of them showed however, can only be obtained by tests of liver signs of hepatobiliary disease. A follow up function tests such as transaminases, alkaline study on all 142 patients with abnormal liver phosphatases, and bilirubin made routinely in function and UC was made between 1989 and unselected groups of patients with UC. Because 1991 to evaluate the cause of the liver abnor- the cause of hepatic involvement in UC is mality and to find out if the liver disease had enigmatic it is important to find out if the rate affected the survival rates. At follow up, eight of this complication is increasing, because of patients were reclassified as having Crohn's factors unrelated to UC, or if it mirrors the disease, 60 had developed normal liver func- incidence ofUC in itself. -
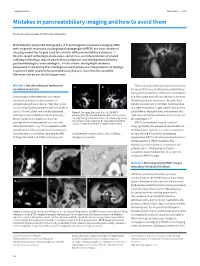
Mistakes in Pancreatobiliary Imaging and How to Avoid Them
ueg education Mistakes in… 2020 Mistakes in pancreatobiliary imaging and how to avoid them Marianna Arvanitakis and Martina Pezzullo Multidetector computed tomography (CT) and magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP) are cross-sectional imaging modalities largely used for patients with pancreatobiliary diseases.1–3 Despite recent technological advances, correct use and interpretation of related radiological findings require good clinical judgment and collaboration between gastroenterologists and radiologists. In this article, we highlight mistakes frequently made during the radiological investigation and interpretation of findings in patients with suspected pancreatobiliary diseases, based on the available literature and on our clinical experience. There are several biliary anatomic variations to Mistake 1 Not describing or looking for a b anatomical variants be aware of that may lead to perioperative biliary injury: perihilar insertion of the cystic duct defined Laparoscopic cholecystectomy is currently as a short cystic duct with an insertion <1 cm from the standard procedure for treatment of the hilum; posterior insertion of the cystic duct symptomatic gallstone disease.4 Bile duct injury into the common bile duct (CBD); direct insertion can occur during the procedure with an incidence of a segmental/sectoral right hepatic duct into the up to 0.7% and, albeit rare, can be associated Figure 1 | Imaging the cystic duct. a | 2D MRCP gallbladder or the cystic duct; and insertion of a with significant morbidity and even mortality.4 showing that what looks like the cystic duct (arrow) is right sectoral/segmental hepatic duct directly into Biliary anatomical variations can lead to actually the right posterior duct separately originating the CBD (figure 1).3,5 from the common bile duct. -

Bile Duct Cancer Causes, Risk Factors, and Prevention Risk Factors
cancer.org | 1.800.227.2345 Bile Duct Cancer Causes, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors for bile duct cancer. ● Bile Duct Risk Factors ● What Causes Bile Duct Cancer? Prevention There's no way to completely prevent cancer. But there are things you can do that might help lower your risk. Learn more. ● Can Bile Duct Cancer Be Prevented? Bile Duct Risk Factors A risk factor is anything that affects your chance of getting a disease like cancer. Different cancers have different risk factors. Some risk factors, like smoking, can be changed. Others, like a person’s age or family history, can’t be changed. But having a risk factor, or even many risk factors, does not mean that a person will get 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 the disease. And many people who get the disease have few or no known risk factors. Researchers have found some risk factors that make a person more likely to develop bile duct cancer. Certain diseases of the liver or bile ducts People who have chronic (long-standing) inflammation of the bile ducts have an increased risk of developing bile duct cancer. Certain conditions of the liver or bile ducts can cause this, these include: ● Primary sclerosing cholangitis (PSC), a condition in which inflammation of the bile ducts (cholangitis) leads to the formation of scar tissue (sclerosis). People with PSC have an increased risk of bile duct cancer. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria.