Lessons from Rhinolophid and Hipposiderid Bats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bat Conservation 2021
Bat Conservation Global evidence for the effects of interventions 2021 Edition Anna Berthinussen, Olivia C. Richardson & John D. Altringham Conservation Evidence Series Synopses 2 © 2021 William J. Sutherland This document should be cited as: Berthinussen, A., Richardson O.C. and Altringham J.D. (2021) Bat Conservation: Global Evidence for the Effects of Interventions. Conservation Evidence Series Synopses. University of Cambridge, Cambridge, UK. Cover image: Leucistic lesser horseshoe bat Rhinolophus hipposideros hibernating in a former water mill, Wales, UK. Credit: Thomas Kitching Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ 3 Contents Advisory Board.................................................................................... 11 About the authors ............................................................................... 12 Acknowledgements ............................................................................. 13 1. About this book ........................................................... 14 1.1 The Conservation Evidence project ................................................................................. 14 1.2 The purpose of Conservation Evidence synopses ............................................................ 14 1.3 Who this synopsis is for ................................................................................................... 15 1.4 Background ..................................................................................................................... -

Morphometrical Variations of Malaysian Hipposideros Species
Malaysian Journal of Mathematical Sciences 6(1): 47-57 (2012) Morphometrical Variations of Malaysian Hipposideros Species Siti Nurlydia Sazali, Charlie J. Laman and M.T. Abdullah Department of Zoology, Faculty of Resource Science and Technology, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia E-mail: [email protected] ABSTRACT A study on the morphometrical variations among four Malaysian Hipposideros species was conducted using voucher specimens deposited in Universiti Malaysia Sarawak (UNIMAS) Zoological Museum and the Department of Widlife and National Park (DWNP) Kuala Lumpur. Twenty two individuals from four species of Hipposideros ater , H. bicolor , H. cineraceus and H. dyacorum were morphologically measured, in which a total of 27 linear parameters of body, skull and dentals of each were appropriately recorded. The statistical data were later subjected to discriminant function analysis (DFA) and canonical variate analysis (CVA) using SPSS version 15.0 and unweighted pair-group method average (UPGMA) cluster analysis using Minitab version 14.4. The highest character loadings observed in Function l, Function 2 and Function 3 were the forearm length (FA), the third digit second phalanx length (D3P2L) and the palatal length (PL) with standardised canonical discriminant function coefficient values of 21.910, 5.770 and 5.095, respectively. These three characters were identified as the best diagnostic features for discriminating these closely related species of Hipposideros . Hence, this morphometric approach could be a promising tool as an alternative to the molecular DNA analysis for identification of Chiroptera species. Keywords: Hipposideros , morphometric, discriminant function analysis cluster analysis, species identification. 1. INTRODUCTION Bats belong to the order Chiroptera and can be distinguished from all other mammals by their ability to fly, which is a result of the modification of their forelimbs into wings (Payne et al . -

Bat Echolocation Research a Handbook for Planning and Conducting Acoustic Studies Second Edition
Bat Echolocation Research A handbook for planning and conducting acoustic studies Second Edition Erin E. Fraser, Alexander Silvis, R. Mark Brigham, and Zenon J. Czenze EDITORS Bat Echolocation Research A handbook for planning and conducting acoustic studies Second Edition Editors Erin E. Fraser, Alexander Silvis, R. Mark Brigham, and Zenon J. Czenze Citation Fraser et al., eds. 2020. Bat Echolocation Research: A handbook for planning and conducting acoustic studies. Second Edition. Bat Conservation International. Austin, Texas, USA. Tucson, Arizona 2020 This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License ii Table of Contents Table of Figures ....................................................................................................................................................................... vi Table of Tables ........................................................................................................................................................................ vii Contributing Authors .......................................................................................................................................................... viii Dedication…… .......................................................................................................................................................................... xi Foreword…….. .......................................................................................................................................................................... -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Keshav Ravi by Keshav Ravi
by Keshav Ravi by Keshav Ravi Preface About the Author In the whole world, there are more than 30,000 species Keshav Ravi is a caring and compassionate third grader threatened with extinction today. One prominent way to who has been fascinated by nature throughout his raise awareness as to the plight of these animals is, of childhood. Keshav is a prolific reader and writer of course, education. nonfiction and is always eager to share what he has learned with others. I have always been interested in wildlife, from extinct dinosaurs to the lemurs of Madagascar. At my ninth Outside of his family, Keshav is thrilled to have birthday, one personal writing project I had going was on the support of invested animal advocates, such as endangered wildlife, and I had chosen to focus on India, Carole Hyde and Leonor Delgado, at the Palo Alto the country where I had spent a few summers, away from Humane Society. my home in California. Keshav also wishes to thank Ernest P. Walker’s Just as I began to explore the International Union for encyclopedia (Walker et al. 1975) Mammals of the World Conservation of Nature (IUCN) Red List species for for inspiration and the many Indian wildlife scientists India, I realized quickly that the severity of threat to a and photographers whose efforts have made this variety of species was immense. It was humbling to then work possible. realize that I would have to narrow my focus further down to a subset of species—and that brought me to this book on the Endangered Mammals of India. -

Bat Coronavirus in the Western Indian Ocean
bioRxiv preprint doi: https://doi.org/10.1101/742866; this version posted September 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Bat coronavirus phylogeography in the western Indian Ocean 2 Running title: Bat coronavirus in the western Indian Ocean 3 Authors: Léa Joffrin*, Steven M. Goodman, David A. Wilkinson, Beza Ramasindrazana, Er- 4 wan Lagadec, Yann Gomard, Gildas Le Minter, Andréa Dos Santos, M. Corrie Schoeman, Ra- 5 jendraprasad Sookhareea, Pablo Tortosa, Simon Julienne, Eduardo S. Gudo, Patrick Mavingui 6 and Camille Lebarbenchon 7 8 Author affiliations : 9 Université de La Réunion, UMR Processus Infectieux en Milieu Insulaire Tropical (PIMIT) 10 INSERM 1187, CNRS 9192, IRD 249, Sainte-Clotilde, La Réunion, France (L. Joffrin, B. Ra- 11 masindrazana, E. Lagadec, Y. Gomard, G. Le Minter, D.A. Wilkinson, P. Tortosa, P. Mavingui, 12 C. Lebarbenchon) 13 Association Vahatra, Antananarivo, Madagascar (S.M. Goodman, B. Ramasindrazana) 14 Field Museum of Natural History, Chicago, USA (S.M. Goodman) 15 Veterinary Faculty, Eduardo Mondlane University, Maputo, Mozambique (A. Dos Santos) 16 School of Life Sciences, University of Kwa-Zulu Natal, Kwa-Zulu Natal, South Africa (M.C. 17 Schoeman) 18 National Parks and Conservation Service, Réduit, Mauritius (R. Sookhareea) 19 Seychelles Ministry of Health, Victoria, Mahe, Seychelles (S. Julienne) 20 Instituto Nacional de Saúde, Maputo, Mozambique (E.S. Gudo). 21 22 *Corresponding author: [email protected] 23 UMR PIMIT, 2 rue Maxime Rivière, 97490 Sainte-Clotilde, Reunion Island, France. 24 Tel: +262 262 93 88 00 1 bioRxiv preprint doi: https://doi.org/10.1101/742866; this version posted September 4, 2019. -
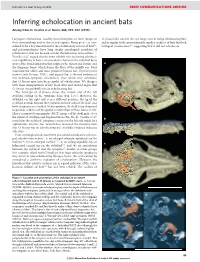
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

African Bat Conservation News
Volume 35 African Bat Conservation News August 2014 ISSN 1812-1268 © ECJ Seamark, 2009 (AfricanBats) Above: A male Cape Serotine Bat (Neoromicia capensis) caught in the Chitabi area, Okavango Delta, Botswana. Inside this issue: Research and Conservation Activities Presence of paramyxo and coronaviruses in Limpopo caves, South Africa 2 Observations, Discussions and Updates Recent changes in African Bat Taxonomy (2013-2014). Part II 3 Voucher specimen details for Bakwo Fils et al. (2014) 4 African Chiroptera Report 2014 4 Scientific contributions Documented record of Triaenops menamena (Family Hipposideridae) in the Central Highlands of 6 Madagascar Download and subscribe to African Bat Conservation News published by AfricanBats at: www.africanbats.org The views and opinions expressed in articles are no necessarily those of the editor or publisher. Articles and news items appearing in African Bat Conservation News may be reprinted, provided the author’s and newsletter refer- ence are given. African Bat Conservation News August 2014 vol. 35 2 ISSN 1812-1268 Inside this issue Continued: Recent Literature Conferences 7 Published Books / Reports 7 Papers 7 Notice Board Conferences 13 Call for Contributions 13 Research and Conservation Activities Presence of paramyxo- and coronaviruses in Limpopo caves, South Africa By Carmen Fensham Department of Microbiology and Plant Pathology, Faculty of Natural and Agricultural Sciences, University of Pretoria, 0001, Republic of South Africa. Correspondence: Prof. Wanda Markotter: [email protected] Carmen Fensham is a honours excrement are excised and used to isolate any viral RNA that student in the research group of may be present. The identity of the RNA is then determined Prof. -

Hipposideros Vittatus – Striped Leaf-Nosed Bat
Hipposideros vittatus – Striped leaf-nosed Bat Assessment Rationale The species is only known from the northern part of the assessment region (extent of occurrence estimated at 1,419 km2), where it occurs in Pafuri, Kruger National Park. Although it qualifies for Vulnerable D2 based on limited number of locations, there are no plausible threats. While no information exists on population size in the assessment region, it is numerous outside South Africa. Thus we assume the population is fairly large and stable in Kruger National Park. We list this species as Least Concern. Regional population effects: The subpopulations that occur in northern Kruger National Park are part of a population that is continuous across the border occurring throughout most of Zimbabwe and Mozambique. The Melissa Donnelly, iNaturalist species overall is widespread in the rest of Africa. Striped Leaf-nosed Bats have a high wing-loading (Norberg & Rayner 1987), and presumably good dispersal potential, Regional Red List status (2016) Least Concern and thus rescue effects are possible. National Red List status (2004) Not Evaluated Reasons for change Non-genuine change: Distribution New information Although fairly sparse within its distribution, this species Global Red List status (2008) Near Threatened A ranges through much of southern, Central and East Africa. The northeastern extent of its range extends from Ethiopia TOPS listing (NEMBA) (2007) None and Somalia to Kenya, Tanzania, Malawi, Zambia and CITES listing None Mozambique. It has a patchy distribution through Central Africa in the Democratic Republic of Congo, Central Endemic No African Republic, Angola, and spreads westwards to Nigeria and Guinea. The southern portion of its Sexual dimorphism is evident in this species; distribution includes Zimbabwe, Botswana, Namibia and apart from the differences in colouring, females the extreme northeastern regions of South Africa. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam
viruses Article Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam Satoru Arai 1, Fuka Kikuchi 1,2, Saw Bawm 3 , Nguyễn Trường Sơn 4,5, Kyaw San Lin 6, Vương Tân Tú 4,5, Keita Aoki 1,7, Kimiyuki Tsuchiya 8, Keiko Tanaka-Taya 1, Shigeru Morikawa 9, Kazunori Oishi 1 and Richard Yanagihara 10,* 1 Infectious Disease Surveillance Center, National Institute of Infectious Diseases, Tokyo 162-8640, Japan; [email protected] (S.A.); [email protected] (F.K.); [email protected] (K.A.); [email protected] (K.T.-T.); [email protected] (K.O.) 2 Department of Chemistry, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan 3 Department of Pharmacology and Parasitology, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; [email protected] 4 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi, Vietnam; [email protected] (N.T.S.); [email protected] (V.T.T.) 5 Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Hanoi, Vietnam 6 Department of Aquaculture and Aquatic Disease, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; [email protected] 7 Department of Liberal Arts, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan 8 Laboratory of Bioresources, Applied Biology Co., Ltd., Tokyo 107-0062, Japan; [email protected] 9 Department of Veterinary Science, National Institute of Infectious Diseases, Tokyo 162-8640, Japan; [email protected] 10 Pacific Center for Emerging Infectious Diseases Research, John A. -

Diversity, Host Specialization, and Geographic Structure of Filarial Nematodes Infecting Malagasy Bats
RESEARCH ARTICLE Diversity, Host Specialization, and Geographic Structure of Filarial Nematodes Infecting Malagasy Bats Beza Ramasindrazana1,2,3*, Koussay Dellagi1,2, Erwan Lagadec1,2, Milijaona Randrianarivelojosia4, Steven M. Goodman3,5, Pablo Tortosa1,2 1 Centre de Recherche et de Veille sur les maladies émergentes dans l’Océan Indien, Plateforme de Recherche CYROI, Sainte Clotilde, La Réunion, France, 2 Université de La Réunion, UMR PIMIT "Processus Infectieux en Milieu Insulaire Tropical", INSERM U 1187, CNRS 9192, IRD 249. Plateforme de Recherche CYROI, 97490 Sainte Clotilde, Saint-Denis, La Réunion, France, 3 Association Vahatra, Antananarivo, Madagascar, 4 Institut Pasteur de Madagascar, Antananarivo, Madagascar, 5 The Field Museum of Natural History, Chicago, Illinois, United States of America * [email protected] OPEN ACCESS Abstract Citation: Ramasindrazana B, Dellagi K, Lagadec E, We investigated filarial infection in Malagasy bats to gain insights into the diversity of these Randrianarivelojosia M, Goodman SM, Tortosa P (2016) Diversity, Host Specialization, and Geographic parasites and explore the factors shaping their distribution. Samples were obtained from Structure of Filarial Nematodes Infecting Malagasy 947 individual bats collected from 52 sites on Madagascar and representing 31 of the 44 Bats. PLoS ONE 11(1): e0145709. doi:10.1371/ species currently recognized on the island. Samples were screened for the presence of journal.pone.0145709 micro- and macro-parasites through both molecular and morphological approaches. Phylo- Editor: Karen E. Samonds, Northern Illinois genetic analyses showed that filarial diversity in Malagasy bats formed three main groups, University, UNITED STATES the most common represented by Litomosa spp. infecting Miniopterus spp. (Miniopteridae); Received: April 30, 2015 a second group infecting Pipistrellus cf.