The Morphology of the Hypoglossal Canal and Its Size in Relation to Skull Capacity in Man and Other Mammal Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Morphometric Study of Hypoglossal Canal of Occipital Bone in Dry Skulls of Two States in Southern Nigeria Enaohwo, Taniyohwo.MAMERHI1, Okoro
Bangladesh Journal of Medical Science Vol. 19 No. 04 October’20 Original article: Morphometric study of hypoglossal canal of occipital bone in dry skulls of two states in southern nigeria Enaohwo, Taniyohwo.MAMERHI1, Okoro. Ogheneyebrorue. GODSWILL Abstract: Background: It is observed that the morphologic and morphometric variability of the occipital bone structures may coexist in the same individual or among different subjects of the same or different populations and thus, a sound knowledge of the morphometry of this area can provide important benefits in determining safe surgical zones during surgical procedures. Aim: The present study was aimed at measuring the length (right and left) and width (right and left) of the hypoglossal canal among adult dry skulls of two states in southern Nigeria. Materials and Method: This study adopted the cross sectional study design. A total of eighty (80) hypoglossal canal; right and left were selected by simple random sampling and their length and width were measured with the aid of the digital vernier caliper. Results: The hypoglossal canal length on the right side was seen to be higher compared to the left length of the hypoglossal canal while the right hypoglossal canal width was seen to be higher compared to the left hypoglossal canal width and also observed differences between the right and left sides were statistically significant (P=0.01). Conclusion: There was a statistical significant difference with regard to hypoglossal canal length (right and left) and width (right and left) among the studied population. Keywords: Occipital bone, hypoglossal canal, morphology, variation Bangladesh Journal of Medical Science Vol. -
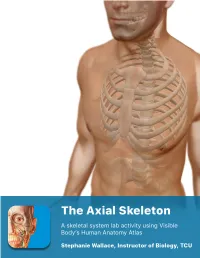
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -
Pseudoforamina of the Skull Base: a Normal Variant
AJNR:8, July/August 1987 CORRESPONDENCE 737 6. Stimac GK, Solomon MA, Newton TH . CT and MR of angiomatous its relative lucency accounts for the apparent opening of the pseu malformations of the choroid plexus in patients with Sturge-Weber disease. doforamen. AJNR 1986 ;7 :623-627 CT of the region of the foramen magnum in a dried skull also demonstrates the presence of the pseudoforamina (Fig . 3), but only at certain gantry angulations, which vary for each patient. Pseudoforamina of the Skull Base: A Normal Correlation of radiographic features of the pseudoforamina in both Variant dried skull and clinical case material demonstrates the anatomic basis for this normal variant. Recognition of its benign nature is vital to Radiologic evaluation of basal foramina of the skull is frequently a avoid diagnostic error in evaluation of the skull base. critical aspect in the diagnosis of patients with deficits referable to cranial nerves. Commonly encountered normal asymmetries and Robert W. Hurst individual variations often make interpretation difficult. A pseudofor Wayne S. Cail amen in the skull base was initially observed in skull radiographs of Thomas Lee Pope, Jr. several patients and correlated with images of a dried skull. We stress Theodore E. Keats the importance of recognizing pseudoforamina to avoid diagnostic University of Virginia Medical Center confusion and error. Charlottesville, VA 22908 Bilateral, rounded lucent areas with sclerotic margins may be Chris Cimmino visualized on submentovertex or Water's views of the skull base. Mary Washington Hospital Located anteromedially to the hypoglossal canals and directly medial Fredericksburg, VA 22401 to the jugular foramen , these structures may be mistaken for the hypoglossal canal , erosion of the jugular foramen, or other normal REFERENCE structures (Fig . -

MBB: Head & Neck Anatomy
MBB: Head & Neck Anatomy Skull Osteology • This is a comprehensive guide of all the skull features you must know by the practical exam. • Many of these structures will be presented multiple times during upcoming labs. • This PowerPoint Handout is the resource you will use during lab when you have access to skulls. Mind, Brain & Behavior 2021 Osteology of the Skull Slide Title Slide Number Slide Title Slide Number Ethmoid Slide 3 Paranasal Sinuses Slide 19 Vomer, Nasal Bone, and Inferior Turbinate (Concha) Slide4 Paranasal Sinus Imaging Slide 20 Lacrimal and Palatine Bones Slide 5 Paranasal Sinus Imaging (Sagittal Section) Slide 21 Zygomatic Bone Slide 6 Skull Sutures Slide 22 Frontal Bone Slide 7 Foramen RevieW Slide 23 Mandible Slide 8 Skull Subdivisions Slide 24 Maxilla Slide 9 Sphenoid Bone Slide 10 Skull Subdivisions: Viscerocranium Slide 25 Temporal Bone Slide 11 Skull Subdivisions: Neurocranium Slide 26 Temporal Bone (Continued) Slide 12 Cranial Base: Cranial Fossae Slide 27 Temporal Bone (Middle Ear Cavity and Facial Canal) Slide 13 Skull Development: Intramembranous vs Endochondral Slide 28 Occipital Bone Slide 14 Ossification Structures/Spaces Formed by More Than One Bone Slide 15 Intramembranous Ossification: Fontanelles Slide 29 Structures/Apertures Formed by More Than One Bone Slide 16 Intramembranous Ossification: Craniosynostosis Slide 30 Nasal Septum Slide 17 Endochondral Ossification Slide 31 Infratemporal Fossa & Pterygopalatine Fossa Slide 18 Achondroplasia and Skull Growth Slide 32 Ethmoid • Cribriform plate/foramina -

Topographical Anatomy and Morphometry of the Temporal Bone of the Macaque
Folia Morphol. Vol. 68, No. 1, pp. 13–22 Copyright © 2009 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl Topographical anatomy and morphometry of the temporal bone of the macaque J. Wysocki 1Clinic of Otolaryngology and Rehabilitation, II Medical Faculty, Warsaw Medical University, Poland, Kajetany, Nadarzyn, Poland 2Laboratory of Clinical Anatomy of the Head and Neck, Institute of Physiology and Pathology of Hearing, Poland, Kajetany, Nadarzyn, Poland [Received 7 July 2008; Accepted 10 October 2008] Based on the dissections of 24 bones of 12 macaques (Macaca mulatta), a systematic anatomical description was made and measurements of the cho- sen size parameters of the temporal bone as well as the skull were taken. Although there is a small mastoid process, the general arrangement of the macaque’s temporal bone structures is very close to that which is observed in humans. The main differences are a different model of pneumatisation and the presence of subarcuate fossa, which possesses considerable dimensions. The main air space in the middle ear is the mesotympanum, but there are also additional air cells: the epitympanic recess containing the head of malleus and body of incus, the mastoid cavity, and several air spaces on the floor of the tympanic cavity. The vicinity of the carotid canal is also very well pneuma- tised and the walls of the canal are very thin. The semicircular canals are relatively small, very regular in shape, and characterized by almost the same dimensions. The bony walls of the labyrinth are relatively thin. -
The Axial Skeleton Visual Worksheet
Biology 201: The Axial Skeleton 1) Fill in the table below with the name of the suture that connects the cranial bones. Suture Cranial Bones Connected 1) Coronal suture Frontal and parietal bones 2) Sagittal suture Left and right parietal bones 3) Lambdoid suture Occipital and parietal bones 4) Squamous suture Temporal and parietal bones Source Lesson: Cranial Bones of the Skull: Structures & Functions 2) Fill in the table below with the name of the bony opening associated with the specific nerve or blood vessel. Bones and Foramina Associated Blood Vessels and/or Nerves Frontal Bone 1) Supraorbital foramen Ophthalmic nerve, supraorbital nerve, artery, and vein Temporal Bone 2) Carotid canal Internal carotid artery 3) Jugular foramen Internal jugular vein, glossopharyngeal nerve, vagus nerve, accessory nerve (Cranial nerves IX, X, XI) Occipital Bone 4) Foramen magnum Spinal cord, accessory nerve (Cranial nerve XI) 5) Hypoglossal canal Hypoglossal nerve (Cranial nerve XII) Sphenoid Bone 6) Optic canal Optic nerve, ophthalmic artery Source Lesson: Cranial Bones of the Skull: Structures & Functions 3) Label the anterior view of the skull below with its correct feature. Frontal bone Palatine bone Ethmoid bone Nasal septum: Perpendicular plate of ethmoid bone Sphenoid bone Inferior orbital fissure Inferior nasal concha Maxilla Orbit Vomer bone Supraorbital margin Alveolar process of maxilla Middle nasal concha Inferior nasal concha Coronal suture Mandible Glabella Mental foramen Nasal bone Parietal bone Supraorbital foramen Orbital canal Temporal bone Lacrimal bone Orbit Alveolar process of mandible Superior orbital fissure Zygomatic bone Infraorbital foramen Source Lesson: Facial Bones of the Skull: Structures & Functions 4) Label the right lateral view of the skull below with its correct feature. -
Osseous Variations of the Hypoglossal Canal Area Published: 2009.03.01 Authors’ Contribution: Georgios K
© Med Sci Monit, 2009; 15(3): BR75-83 WWW.MEDSCIMONIT.COM PMID: 19247236 Basic Research BR Received: 2008.01.08 Accepted: 2008.03.31 Osseous variations of the hypoglossal canal area Published: 2009.03.01 Authors’ Contribution: Georgios K. Paraskevas1ADE, Parmenion P. Tsitsopoulos2BEF, A Study Design Basileios Papaziogas1AC, Panagiotis Kitsoulis1CD, Sofi a Spanidou1D, B Data Collection 2 C Statistical Analysis Philippos Tsitsopoulos AD D Data Interpretation E Manuscript Preparation 1 Department of Human Anatomy, Aristotle University of Thessaloniki, Thessaloniki, Greece F Literature Search 2 Department of Neurosurgery, Hippokration General Hospital, Aristotle University of Thessaloniki, Thessaloniki, G Funds Collection Greece Source of support: Self fi nancing Summary Background: The hypoglossal canal is a paired bone passage running from the posterior cranial fossa to the na- sopharyngeal carotid space. Hyperostotic variations of this structure have been described. Material/Methods: One hundred sixteen adult cadaveric dried skull specimens were analyzed. Several canal features, dimensions, and distances relative to constant and reliable landmarks were recorded. Results: One osseous spur in the inner or outer orifi ce of the canal was present in 18.10% of specimens (42/232). Two or more osseous spurs were evident in 0.86% of specimens (2/232). However, com- plete osseous bridging, in the outer or inner part of the canal, was evident in 19.83% of specimens (46/232). Osseous bridging extending through the entire course of the canal was visible in 1.72% of the specimens (4/232). The mean lateral length of the canal was 10.22 mm, the mean medial length was 8.93 mm, the mean transverse and vertical diameters of the internal orifi ce were 7.44 mm and 4.42 mm, respectively, and the mean transverse and vertical diameters of the external or- ifi ce were 6.15 mm and 3.91 mm, respectively. -

The Midline Suboccipital Subtonsillar Approach to the Hypoglossal Canal: Surgical Anatomy and Clinical Application
Acta Neurochir (Wien) (2006) 148: 965–969 DOI 10.1007/s00701-006-0816-3 Neurosurgical Technique The midline suboccipital subtonsillar approach to the hypoglossal canal: surgical anatomy and clinical application M. Tatagiba, A. Koerbel, and F. Roser Department of Neurosurgery, University of Tuubingen,€ Tuubingen,€ Germany Received October 26, 2005; accepted May 4, 2006; published online July 5, 2006 # Springer-Verlag 2006 Summary hypoglossal canal. Because hypoglossal schwannomas Primary lesions of the hypoglossal canal, such as hypoglossal schwan- usually produces enlargement of the hypoglossal canal nomas, are rare. No consensus exists with regard to the surgical approach the straight angle of view given by the STA allows the of choice for treatment of these lesions. Usually, lateral transcondylar surgeon to follow the tumour along to its intracanalicular approaches have been used. The authors describe the surgical anatomy portion up to the extracranial spaces. An example of a of the midline subtonsillar approach to the hypoglossal canal. This ap- proach includes a midline suboccipital craniotomy, dorsal opening of the large hypoglossal schwannoma is shown to illustrate the foramen magnum and elevation of ipsilateral cerebellar tonsil to expose technical details of the STA. the hypoglossal nerve and its canal. The midline subtonsillar approach permits a straight primary intradural view to the hypoglossal canal. There is no necessity of condylar resections. The surgical anatomy of the sub- Anatomy tonsillar approach is described and illustrated by an example of a case. Keywords: Hypoglossal canal; schwannoma; foramen magnum; The hypoglossal nerve consists intracranially of three approach; skull base; posterior fossa. portions: the intramedullary, the cisternal and the can- alicular segment. -

The Axial Skeleton Visual Worksheet
Biology 201: The Axial Skeleton 1) Fill in the table below with the name of the suture that connects the cranial bones. Suture Cranial Bones Connected 1) Frontal and parietal bones 2) Left and right parietal bones 3) Occipital and parietal bones 4) Temporal and parietal bones Source Lesson: Cranial Bones of the Skull: Structures & Functions 2) Fill in the table below with the name of the bony opening associated with the specific nerve or blood vessel. Bones and Foramina Associated Blood Vessels and/or Nerves Frontal Bone 1) Ophthalmic nerve, supraorbital nerve, artery, and vein Temporal Bone 2) Internal carotid artery 3) Internal jugular vein, glossopharyngeal nerve, vagus nerve, accessory nerve (Cranial nerves IX, X, XI) Occipital Bone 4) Spinal cord, accessory nerve (Cranial nerve XI) 5) Hypoglossal nerve (Cranial nerve XII) Sphenoid Bone 6) Optic nerve, ophthalmic artery Source Lesson: Cranial Bones of the Skull: Structures & Functions 3) Label the anterior view of the skull below with its correct feature. Frontal bone Palatine bone Ethmoid bone Nasal septum: Perpendicular plate of ethmoid bone Sphenoid bone Inferior orbital fissure Inferior nasal concha Maxilla Orbit Vomer bone Supraorbital margin Alveolar process of maxilla Middle nasal concha Inferior nasal concha Coronal suture Mandible Glabella Mental foramen Nasal bone Parietal bone Supraorbital foramen Orbital canal Temporal bone Lacrimal bone Orbit Alveolar process of mandible Superior orbital fissure Zygomatic bone Infraorbital foramen Source Lesson: Facial Bones of the Skull: -

The Posterior Condylar Canal
The Posterior Condylar Canal Lawrence E. Ginsberg PURPOSE: To assess the prevalence and appearance of the posterior condylar canal using high resolution CT. METHODS: One hundred twenty-three high-resolution temporal bone CT exami nations were retrospectively reviewed for the presence or absence of the posterior condylar canal. Thirty-four gross skulls were also examined. RESULTS: The posterior condylar canal was identified on CT bilaterally in 31 % of the final study group and unilaterally in 50%. On gross specimens, this structure was identified in 55.9% bilaterally and 17.6% unilaterally. The posterior condylar canal, when present, is readily identifiable in a predictable location. The imaging appearance of this structure is dependent on its relationship to the angle of scanning. CONCLUSION: The posterior condylar canal is among the largest emissary foramina in the human skull. Recognition of this structure and its role as an alternative source of venous drainage from the brain will help avoid misinterpretation during CT examination. Index terms: Skull, anatomy; Skull, computed tomography; Cerebral blood flow AJNR Am J Neuroradio/15:969-972, May 1994 Emissary veins exist in the skull as an escape tween the jugular bulb and the suboccipital ve mechanism, an alternative route of egress for nous plexus (1-4). venous blood (1-4). When the normal routes of The purpose of this report was to study the venous drainage are patent, the role of emissary anatomy and imaging appearance of the posterior veins is limited. However, should venous outflow condyloid canal. Recognition of this common be compromised (ie, jugular thrombosis), these canal is useful for understanding alternative path accessory conduits become an important alter ways of venous flow and avoiding confusion native pathway for venous drainage of the brain. -

THE JOURNAL of NUCLEAR MEDICINE • Vol. 60 • No. 3 • March 2019 Lee Et Al
SUPPLEMENTAL FIGURE 1. Illustration of the nerves commonly involved in perineural spread. The trigeminal nerve and its three divisions (A), the facial nerve and its five terminal motor branches (B), and the hypoglossal nerve (C) are shown on a lateral skull radiograph. THE JOURNAL OF NUCLEAR MEDICINE • Vol. 60 • No. 3 • March 2019 Lee et al. SUPPLEMENTAL FIGURE 2. Bony pathways used by the nerves commonly involved in perineural spread. The maxillary and mandibular divisions of the trigeminal nerve exit the skull through foramen rotundum and foramen ovale, respectively (A). The facial nerve traverses the internal acoustic canal (B) before exiting the skull through the stylomastoid foramen. The ophthalmic division of the trigeminal nerve enters the orbit by via the superior orbital fissure (B). The hypoglossal nerve travels through the hypoglossal canal (A) to enter the carotid space. The glossopharyngeal nerve, vagus nerve, and spinal accessory nerve enter the carotid space via the jugular foramen (A). THE JOURNAL OF NUCLEAR MEDICINE • Vol. 60 • No. 3 • March 2019 Lee et al. SUPPLEMENTAL FIGURE 3. Cranial nerve I involvement in a patient with poorly differentiated carcinoma of the left sinonasal cavity and hypermetabolic tumor spread through the left cribriform plate (arrow) on coronal 18F-FDG PET image (A). Enhancing mass in the left sinonasal cavity invading through the left cribriform plate is seen with left olfactory bulb involvement (arrow) with normal right olfactory bulb (arrowhead) on coronal T1-weighted fat- suppressed post-contrast MRI (B). The tumor was inoperable due to its extensive invasion into the surrounding structures. THE JOURNAL OF NUCLEAR MEDICINE • Vol.