Dark Fermentation Optimization by Anaerobic Digested Sludge Recirculation: Effects on Hydrogen Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fermentative Hydrogen Production - Process Design and Bioreactors
Chemical and Process Engineering 2012, 33 (4), 585-594 DOI: 10.2478/v10176-012-0048-4 FERMENTATIVE HYDROGEN PRODUCTION - PROCESS DESIGN AND BIOREACTORS Małgorzata Waligórska* A. Mickiewicz University, Faculty of Chemistry, ul. Umultowska 89b, 61-614 Poznań, Poland Substitution of fossil fuels with alternative energy carriers has become necessary due to climate change and fossil fuel shortages. Fermentation as a way of producing biohydrogen, an attractive and environmentally friendly future energy carrier, has captured received increasing attention in recent years because of its high H2 production rate and a variety of readily available waste substrates used in the process. This paper discusses the state-of-the-art of fermentative biohydrogen production, factors affecting this process, as well as various bioreactor configurations and performance parameters, including H2 yield and H2 production rate. Keywords: biohydrogen production, fermentation, bioreactors, operational parameters, hydrogen yields 1. INTRODUCTION Energy is a factor significantly influencing the development of civilization. In 2010, its world consumption rose by 5.6 % compared to previous year and equaled 12,002.4 mln tonnes oil equivalent. 87 % of the used energy was generated from fossil fuels (33.5 % crude oil, 29.6 % coal, 23.8 % natural gas) (Statistical Review of World Energy, 2011). If the global population increases by 1.4 billion people over the next 20 years, and assuming an economic growth rate of 3.7%, it has been assessed that the global energy demand will rise by 39 % by 2030 (Statistical Review of World Energy, 2011), whereas in 2100, it can reach a value 3.5 times higher than nowadays (Kruse et al., 2005). -

Hydrogen Production by Anaerobic Fermentation Using Agricultural and Food Processing Wastes Utilizing a Two-Stage Digestion System
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 12-2008 Hydrogen Production By Anaerobic Fermentation Using Agricultural and Food Processing Wastes Utilizing a Two-Stage Digestion System Reese S. Thompson Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Environmental Engineering Commons Recommended Citation Thompson, Reese S., "Hydrogen Production By Anaerobic Fermentation Using Agricultural and Food Processing Wastes Utilizing a Two-Stage Digestion System" (2008). All Graduate Theses and Dissertations. 208. https://digitalcommons.usu.edu/etd/208 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. HYDROGEN PRODUCTION BY ANAEROBIC FERMENTATION USING AGRICULTURAL AND FOOD PROCESSING WASTES UTILIZING A TWO-STAGE DIGESTION SYSTEM by Reese S. Thompson A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biological Engineering Approved: _______________________ _______________________ Dr. Conly L. Hansen Dr. Carl S. Hansen Major Professor Committee Member _______________________ _______________________ Dr. Sridhar Viamajala Dr. Byron Burnhan Committee Member Dean of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2008 ii Copyright © Reese S. Thompson 2008 All Rights Reserved iii ABSTRACT Hydrogen Production by Anaerobic Fermentation Using Agricultural and Food Processing Wastes Utilizing a Two-Stage Digestion System by Reese S. Thompson, Master of Science Utah State University, 2008 Major Professor: Dr. Conly L. Hansen Department: Biological and Irrigation Engineering Hydrogen production by means of anaerobic fermentation was researched utilizing three different substrates. -

Hydrogen Production by Dark Fermentation
481 A publication of CHEMICAL ENGINEERINGTRANSACTIONS The Italian Association VOL. 38, 2014 of Chemical Engineering www.aidic.it/cet GuestEditors: Enrico Bardone, Marco Bravi, Taj Keshavarz Copyright © 2014, AIDIC ServiziS.r.l., ISBN 978-88-95608-29-7; ISSN 2283-9216 DOI: 10.3303/CET1438081 Hydrogen Production by Dark Fermentation VicelmaL. Cardoso, Betânia B. Romão*, Felipe T. M. Silva, Júlia G. Santos, Fabiana R. X. Batista, Juliana S. Ferreira Universidade Federal de Uberlânidia – Faculdade de Engenharia Química. Avenida João Naves de Ávila, 2121, Bloco 1K, Campus Santa Mônica, Uberlândia-MG, Brazil, Zip code: 38408-100 [email protected] Alternative energy sources have been extensively studied due to the environmental concerns caused by the employment of fossil fuels. Hydrogen is considered a very clean energy source, since its combustion releases mainly water as a reaction product and also it has the advantage of having the highest energy density when compared to any other fuel. Moreover, hydrogen may be produced biologically by fermentation from renewable sources, such as effluents and agroindustrial wastes. Based on this context, this work evaluated the hydrogen production by dark fermentation, using a microbial consortium obtained from a dairy wastewater treatment plant. The fermentative process occurred in batch with a reaction volume of 75 mL using lactose (20g/L) as substrate, which was procured from whey permeate. The response hydrogen conversion was evaluated from the process variables, temperature, which ranged from 27,9 to 42°C, and magnesium sulfate concentration, which varied from 0,31 to 1,44 g/L. The response surface, which was plotted from the results obtained in the Central Composite Design pointed out for best hydrogen yield for the temperature range from 30ºC to 35ºC and MgSO4 from 1.2 to 1.6 g/L. -

Producing Hydrogen in Sequential Dark and Photofermentation from Four Different Distillery Wastewaters
Pol. J. Environ. Stud. Vol. 29, No. 4 (2020), 2935-2944 DOI: 10.15244/pjoes/112062 ONLINE PUBLICATION DATE: 2020-03-02 Original Research Producing Hydrogen in Sequential Dark and Photofermentation from Four Different Distillery Wastewaters Roman Zagrodnik*, Krystyna Seifert, Mikołaj Stodolny, Anna Juszczak Faculty of Chemistry, Adam Mickiewicz University, Poznań, Poland Received: 1 August 2019 Accepted: 1 September 2019 Abstract Four different distillery wastewaters were applied as the substrates for dark and photofermentation – wheat stillage (WTL), wheat syrup (WSR), maize stillage (MTL) and maize syrups (MSR). WTL was found to be the most effective substrate in dark fermentation, with a hydrogen production of 0.88 L H2/Lmedium and a yield of 1.17 L H2/LWTL. In the photofermentation experiments, the highest hydrogen production (1.7 L H2/Lmedium) and the highest yield (8.6 L H2/LWSR) were obtained with WSR as the substrate. A sequential two-step treatment, comprising dark fermentation followed by photofermentation, was applied to the same four substrates. WTL and MTL were the substrates for dark fermentation and the effluent after 5-fold dilution was used as the substrate in photofermentation. The maximum hydrogen production in the two-step system was 4.47 L H2/LWTL. Simultaneously, the organic compounds from the waste and those formed in dark fermentation were completely utilized, except for ethanol and methanol, which were 19% and 10% utilized, respectively. Keywords: distillery wastewaters, dark fermentation, photofermentation, biohydrogen, two-step system Introduction was estimated that the world demand for ethyl alcohol would increase by 70% in the next 10 years because of Large-scale fermentative production of ethyl its application as a gasoline component [1]. -

Improving Biohydrogen Productivity by Microbial Dark- and Photo- MARK Fermentations: Novel Data and Future Approaches ⁎ Karen Trchouniana,B, R
Renewable and Sustainable Energy Reviews 80 (2017) 1201–1216 Contents lists available at ScienceDirect Renewable and Sustainable Energy Reviews journal homepage: www.elsevier.com/locate/rser Improving biohydrogen productivity by microbial dark- and photo- MARK fermentations: Novel data and future approaches ⁎ Karen Trchouniana,b, R. Gary Sawersc, Armen Trchouniana,b, a Research Institute of Biology, Faculty of Biology, Yerevan State University, Armenia b Department of Biochemistry, Microbiology and Biotechnology, Faculty of Biology, Yerevan State University, 0025 Yerevan, Armenia c Institute of Biology/Microbiology, Martin-Luther University of Halle-Wittenberg, Halle (Saale), Germany ARTICLE INFO ABSTRACT Keywords: Hydrogen (H2)isaneffective, environmentally friendly and renewable source of fuel that can be produced Hydrogen production during dark- and photo-fermentation by different facultative and obligate anaerobic and purple bacteria and Dark- and photo-fermentation microalgae. This product is known as biohydrogen. It has the advantage of variable yield at low temperature (for Bacteria and microalgae mesophiles growing best at moderate temperature) and relatively low production cost, if compared with Hydrogenases and nitrogenases thermochemical methods. To develop fermentative H production biotechnology using cheap carbonaceous by- Glycerol 2 products and utilization of organic wastes, the selection or construction of effective bacterial strains and Carbon-containing wastes Renewable and sustainable energy optimization of technology process conditions are required. Here we review recent new data that have been obtained with Escherichia coli, Clostridium beijerinskii, Rhodobacter sphaeroides and other bacteria. Activities of [Ni-Fe]-hydrogenases of dark-fermentative bacteria and [Mo]-nitrogenase and [Ni-Fe]-hydrogenase of photo-fermentative species have been examined after growth with different carbon sources, using pure cultures, as well as co-culture and mixed-cultures technologies. -

Hybrid Strategies to Combine Dark Fermentation and Photo
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of Birmingham Research Archive, E-prints Repository Redwood et al. - Dual systems for Bio-H2 – Reviews in Environ. Sci. Bio/Technol. 8:149 1 http://dx.doi.org/10.1007/s11157-008-9144-9 Integrating dark and light bio-hydrogen production strategies: towards the hydrogen economy Mark D. Redwood, Marion Paterson-Beedle and Lynne E. Macaskie School of Biosciences, University of Birmingham Correspondence should be addressed to Dr Mark D. Redwood, School of Biosciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. Tel: +44 1214145889 Fax: +44 1214145925 e-mail: [email protected] Keywords: bio-hydrogen, bioenergy, renewable energy, hydrogen economy, dark fermentation, dual systems, photosynthesis. Abstract Biological methods of hydrogen production are preferable to chemical methods because of the possibility to use sunlight, CO2 and organic wastes as substrates for environmentally benign conversions, under moderate conditions. By combining different microorganisms with different capabilities, the individual strengths of each may be exploited and their weaknesses overcome. Mechanisms of bio-hydrogen production are described and strategies for their integration are discussed. Dual systems can be divided broadly into wholly light-driven systems (with microalgae/cyanobacteria as the 1st stage) and partially light-driven systems (with a dark, fermentative initial reaction). Review and evaluation of published data suggests that the latter type of system holds greater promise for industrial application. This is because the calculated land area required for a wholly light-driven dual system would be too large for either centralised (macro-) or decentralised (micro-)energy generation. -
Hydrogen Production Cost from Fermentation Originator: Katie Randolph and Sarah Studer (DOE)
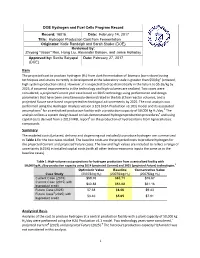
DOE Hydrogen and Fuel Cells Program Record Record: 16016 Date: February 14, 2017 Title: Hydrogen Production Cost f rom Fermentation Originator: Katie Randolph and Sarah Studer (DOE) Reviewed by: Zhiyong "Jason" Ren, Hong Liu, Alexander Beliaev, and Jamie Holladay Approved by: Sunita Satyapal Date: February 27, 2017 (DOE) Item The projected cost to produce hydrogen (H2) from dark fermentation of biomass (corn stover) using techniques and strains currently in development at the laboratory scale is greater than $50/kg1 (untaxed, high system production rates). However, it is expected to drop dramatically in the future to $5.65/kg by 2025, if assumed improvements in the technology and high volumes are realized. Two cases were considered, a projected Current year case based on 2015 technology using performance and design parameters that have been simultaneously demonstrated in the lab at low reactor volumes, and a projected Future case based on projected technological advancements by 2025. The cost analysis was performed using the Hydrogen Analysis version 3.101 (H2A Production v3.101) model and its associated 2 3 assumptions for a centralized production facility with a production capacity of 50,000 kg H2/day. The analysis utilizes a system design based on lab-demonstrated hydrogen production procedures4 and using capital costs derived from a 2013 NREL report5 on the production of hydrocarbons from lignocellulosic compounds. Summary The modeled costs (untaxed, delivery and dispensing not included) to produce hydrogen are summarized in Table 1 for the two cases studied. The baseline costs are the projected costs to produce hydrogen for the projected Current and projected Future cases. -

Dark Fermentation (4 H2 /Glucose + 2 Acetates);
Overview of microbial hydrogen production Carolina Zampol Lazaro Stagiaire postdoctoral – Université de Montréal Supervisor: Prof. Dr. Patrick Hallenbeck Senior Research Associate, National Research Council Department of Biology, US Air Force Academy Reduction of CO2 with Hydrogen Actual hydrogen production: natural gas via steam methane reforming (> 90%) Barrier to overcome: sustainable hydrogen production - electrolysis of water and biomass processing (using a variety of technologies ranging from reforming to fermentation). Biological hydrogen producing microorganisms Great diversity! Metabolic versatility! Source: Chandrasekhar, K., Lee, Y.-J., & Lee, D.-W. (2015). Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. International Journal of Molecular Sciences, 16(4). Overview of the 50-L horizontal tubular photobioreactor Biophotolysis used for outdoor experiments with C. reinhardtii • Abundant substrate = H2O • Abundant energy source = sun light • Simple products: H2 and O2 • Oxygen sensitive hydrogenase • Low light conversion efficiencies Source: Scoma, A., Giannelli, L., Faraloni, C., & Torzillo, G. (2012). Outdoor H(2) production in a 50-L tubular photobioreactor by means of a sulfur-deprived • Expensive hydrogen impermeable culture of the microalga Chlamydomonas reinhardtii. J Biotechnol, 157(4), 620- photobioreactors required 627. Indirect biophotolysis by Nonheterocystous Cyanobacteria • Separation of the H2 and O2 evolution reactions 1- Production of the biomass (carbohydrates) - -

Biohydrogen (Bioh2) Consortium to Advance Fermentative Hydrogen Production
BioHydrogen (BioH2) Consortium to Advance Fermentative Hydrogen Production Pin-Ching Maness (Primary Contact), Katherine • Use a cellulose-degrading microbe to ferment Chou, Lauren Magnusson, and Wei Xiong biomass (without adding enzyme cocktail) to National Renewable Energy Laboratory lower feedstock cost 15013 Denver West Parkway Golden, CO 80401 • Generate an engineered biomass strain, Phone: 303-384-6114 combining the best metabolic features to Email: [email protected] improve hydrogen production rate, molar yield, and biomass utilization, the latter DOE Manager: Katie Randolph lowering biomass feedstock cost Phone: 720-356-1759 Email: [email protected] • Develop bioreactor processes for high-solid loadings that will lower capital expenses Subcontractor(s): • Lawrence Berkeley National Laboratory • Integrate fermentation with MEC to increase • Pacific Northwest National Laboratory overall hydrogen molar yield • Argonne National Laboratory • Conduct TEA, LCA, and strategies for system Project Start Date: October 1, 2018 integration Project End Date: September 30, 2021 • Collectively advance fermentative hydrogen and increase its techno-economic feasibility for scale-up hydrogen production, leveraging Overall Objectives the unique capabilities delivered by the four • Improve the rates and molar yields of National Lab teams. hydrogen production (mol H2/mol sugar) via metabolic engineering of the cellulose Technical Barriers degrader Clostridium thermocellum (National This project addresses technical barriers from Renewable -

Mesophilic and Thermophilic Biohydrogen and Bioelectricity Production from Real and Synthetic Wastewaters Paolo Dessi
Mesophilic and thermophilic biohydrogen and bioelectricity production from real and synthetic wastewaters Paolo Dessi To cite this version: Paolo Dessi. Mesophilic and thermophilic biohydrogen and bioelectricity production from real and synthetic wastewaters. Environmental Engineering. Université Paris-Est; Tampereen yliopisto, 2018. English. NNT : 2018PESC2056. tel-02373735 HAL Id: tel-02373735 https://tel.archives-ouvertes.fr/tel-02373735 Submitted on 21 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Joint PhD degree in Environmental Technology Docteur de l’Université Paris-Est Spécialité : Science et Technique de l’Environnement Dottore di Ricerca in Tecnologie Ambientali Degree of Doctor in Environmental Technology Thesis for the degree of Doctor of Philosophy in Environmental Technology Tesi di Dottorato – Thèse – PhD thesis – Väitöskirja Paolo Dessì Mesophilic and thermophilic biohydrogen and bioelectricity production from real and synthetic wastewaters 23/05/2018, Tampere In front of the PhD evaluation committee Prof. Alan Guwy Reviewer Dr. Serge Hiligsmann Reviewer Prof. Jóhann Örlygsson Reviewer Prof. Piet N.L. Lens Promotor Prof. Giovanni Esposito Co-Promotor Prof. Hab. Eric D. van Hullebusch Co-Promotor Asst. Prof. Aino-Maija Lakaniemi Co-Promotor Prof. Jukka Rintala Chair Marie Sklodowska-Curie European Joint Doctorate, Advanced Biological Waste-to-Energy Technologie Evaluation committee Chair Prof. -

Alternative Methods for Dark Fermentation Course Analysis
Research Article Alternative methods for dark fermentation course analysis Edyta Słupek1 · Karolina Kucharska1 · Jacek Gębicki1 © The Author(s) 2019 OPEN Abstract Dark fermentation course analysis is crucial, as complexed matrix of gaseous components may be formed and revealed during the process. The paper considers key issues related to the microbiological process in which complex organic substances are transformed into hydrogen. For the purposes of hydrogen generation, the application of wastewater mixed sludge pre-treated according to Faloye method (Faloye et al. in Int J Hydrog Energy 38:11765–11773, 2013. https ://doi.org/10.1016/j.ijhyd ene.2013.06.129; Int J Hydrog Energy 39:5607–5616, 2014. https ://doi.org/10.1016/j.ijhyd ene.2014.01.163) was applied. The main risk of by-product formation is related to the presence of methanogens, i.e., Archea, in the sludge. The application of gaseous chromatography confrmed the presence of hydrogen during the initial, lag and log phases of the culture and methane in the late logarithmic death phase of the culture. However, other fermen- tation gaseous products’ presence was not confrmed, as their concentration was under the limit of detection. Therefore, a revision regarding the application of matrix sensors was proposed, and the levels of gases able to be measured using both gas chromatography and matrix sensors were conducted. The criteria of matrix sensors’ selection should include the selectivity not only for the hydrogen, hydrogen sulfde or methane, but also the sensitivity to the response of other gases contained in the mixture—ammonium, carbon dioxide and oxygen. A comprehensive combination of commer- cially available sensors and their applicability for the purposes of dark fermentation course analysis was presented on the basis of the levels of gas concentrations in the generated gas mixture. -

Biohydrogen Production by Sequential Dark and Photofermentation
IOSR Journal of Computer Engineering (IOSRJCE) ISSN: 2278-0661 Volume 1, Issue 4 (May-June 2012), PP 39-47 www.iosrjournals.org Production of Clean Fuel from Waste Biomass using Combined Dark and Photofermentation Hema R, Pushpa Agrawal Dr. M.G.R. Educational and Research Institute University, Chennai Abstract: Sequential dark and photo- fermentation is a rather new approach in biological hydrogen gas production. In the present work, two-stage fermentation process consisting of dark and photo-fermentation periods was carried out in a batch reactor. The study mainly emphasized on assessing the potential of biological conversion of different substrates to hydrogen by studying various parameters like temperature, pH and cell density to achieve maximum hydrogen production. In the first stage, substrate was fermented in the dark stage using Bacillus licheniformis, Enterobacter cloacae and Halobacterium salinarum to produce acetate, CO2 and H2. The acetate produced in the first stage is fermented to H2 and CO2 by Rhodobacter sphaeroides for further hydrogen production in the second, illuminated stage. The percentage yield for Bacillus licheniformis, Enterobacter cloacae and Halobacterium salinarum using Rhodobacter sphaeroides was found to be 35.7%, 32%, and 26% respectively and process proficiency was found to be 0.2, 0.35 and 0.16 moles/kg. Key Words: Hydrogen; Dark fermentation; Photo- fermentation; Bacillus licheniformis, Enterobacter cloacae; Halobacterium salinarum; Rhodobacter sphaeroides I. INTRODUCTION Two-stage system has certain advantages over single- stage dark-fermentation or photo-fermentation processes. The effluent of dark fermentation in hydrogen production provides sufficient amount of organic acids for the photo- fermentation. Therefore, the limitation by the organic acid availability would be eliminated.