Review a Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fermentative Hydrogen Production - Process Design and Bioreactors
Chemical and Process Engineering 2012, 33 (4), 585-594 DOI: 10.2478/v10176-012-0048-4 FERMENTATIVE HYDROGEN PRODUCTION - PROCESS DESIGN AND BIOREACTORS Małgorzata Waligórska* A. Mickiewicz University, Faculty of Chemistry, ul. Umultowska 89b, 61-614 Poznań, Poland Substitution of fossil fuels with alternative energy carriers has become necessary due to climate change and fossil fuel shortages. Fermentation as a way of producing biohydrogen, an attractive and environmentally friendly future energy carrier, has captured received increasing attention in recent years because of its high H2 production rate and a variety of readily available waste substrates used in the process. This paper discusses the state-of-the-art of fermentative biohydrogen production, factors affecting this process, as well as various bioreactor configurations and performance parameters, including H2 yield and H2 production rate. Keywords: biohydrogen production, fermentation, bioreactors, operational parameters, hydrogen yields 1. INTRODUCTION Energy is a factor significantly influencing the development of civilization. In 2010, its world consumption rose by 5.6 % compared to previous year and equaled 12,002.4 mln tonnes oil equivalent. 87 % of the used energy was generated from fossil fuels (33.5 % crude oil, 29.6 % coal, 23.8 % natural gas) (Statistical Review of World Energy, 2011). If the global population increases by 1.4 billion people over the next 20 years, and assuming an economic growth rate of 3.7%, it has been assessed that the global energy demand will rise by 39 % by 2030 (Statistical Review of World Energy, 2011), whereas in 2100, it can reach a value 3.5 times higher than nowadays (Kruse et al., 2005). -

Hydrogen Production by Anaerobic Fermentation Using Agricultural and Food Processing Wastes Utilizing a Two-Stage Digestion System
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 12-2008 Hydrogen Production By Anaerobic Fermentation Using Agricultural and Food Processing Wastes Utilizing a Two-Stage Digestion System Reese S. Thompson Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Environmental Engineering Commons Recommended Citation Thompson, Reese S., "Hydrogen Production By Anaerobic Fermentation Using Agricultural and Food Processing Wastes Utilizing a Two-Stage Digestion System" (2008). All Graduate Theses and Dissertations. 208. https://digitalcommons.usu.edu/etd/208 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. HYDROGEN PRODUCTION BY ANAEROBIC FERMENTATION USING AGRICULTURAL AND FOOD PROCESSING WASTES UTILIZING A TWO-STAGE DIGESTION SYSTEM by Reese S. Thompson A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biological Engineering Approved: _______________________ _______________________ Dr. Conly L. Hansen Dr. Carl S. Hansen Major Professor Committee Member _______________________ _______________________ Dr. Sridhar Viamajala Dr. Byron Burnhan Committee Member Dean of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2008 ii Copyright © Reese S. Thompson 2008 All Rights Reserved iii ABSTRACT Hydrogen Production by Anaerobic Fermentation Using Agricultural and Food Processing Wastes Utilizing a Two-Stage Digestion System by Reese S. Thompson, Master of Science Utah State University, 2008 Major Professor: Dr. Conly L. Hansen Department: Biological and Irrigation Engineering Hydrogen production by means of anaerobic fermentation was researched utilizing three different substrates. -

Hydrogen Production by Dark Fermentation
481 A publication of CHEMICAL ENGINEERINGTRANSACTIONS The Italian Association VOL. 38, 2014 of Chemical Engineering www.aidic.it/cet GuestEditors: Enrico Bardone, Marco Bravi, Taj Keshavarz Copyright © 2014, AIDIC ServiziS.r.l., ISBN 978-88-95608-29-7; ISSN 2283-9216 DOI: 10.3303/CET1438081 Hydrogen Production by Dark Fermentation VicelmaL. Cardoso, Betânia B. Romão*, Felipe T. M. Silva, Júlia G. Santos, Fabiana R. X. Batista, Juliana S. Ferreira Universidade Federal de Uberlânidia – Faculdade de Engenharia Química. Avenida João Naves de Ávila, 2121, Bloco 1K, Campus Santa Mônica, Uberlândia-MG, Brazil, Zip code: 38408-100 [email protected] Alternative energy sources have been extensively studied due to the environmental concerns caused by the employment of fossil fuels. Hydrogen is considered a very clean energy source, since its combustion releases mainly water as a reaction product and also it has the advantage of having the highest energy density when compared to any other fuel. Moreover, hydrogen may be produced biologically by fermentation from renewable sources, such as effluents and agroindustrial wastes. Based on this context, this work evaluated the hydrogen production by dark fermentation, using a microbial consortium obtained from a dairy wastewater treatment plant. The fermentative process occurred in batch with a reaction volume of 75 mL using lactose (20g/L) as substrate, which was procured from whey permeate. The response hydrogen conversion was evaluated from the process variables, temperature, which ranged from 27,9 to 42°C, and magnesium sulfate concentration, which varied from 0,31 to 1,44 g/L. The response surface, which was plotted from the results obtained in the Central Composite Design pointed out for best hydrogen yield for the temperature range from 30ºC to 35ºC and MgSO4 from 1.2 to 1.6 g/L. -

Photobiological Hydrogen Production: Photochemical E Ciency and Bioreactor Design
International Journal of Hydrogen Energy 27 (2002) 1195–1208 www.elsevier.com/locate/ijhydene Photobiological hydrogen production: photochemical e)ciency and bioreactor design Ida Akkermana; ∗, Marcel Janssenb, Jorge Rochac, RenÃe H. Wij1elsd aThe New Delta, Laan 1933-1, 6711 NX Ede, Netherlands bFood and Bioprocess Engineering Group, Wageningen University, Netherlands cChemical Engineering Department, University of Coimbra, Portugal dFood and Bioprocess Engineering Group, Wageningen University, Netherlands Abstract Biological production of hydrogen can be carried out by photoautotrophic or photoheterotrophic organisms. Here, the photosystems of both processes are described. The main drawback of the photoautotrophic hydrogen production process is oxygen inhibition. The few e)ciencies reported on the conversion of light energy into hydrogen energy are low, less than 1.5% on a solar spectrum basis. However, these can be increased to 3–10%, by the immediate removal of produced oxygen. The photochemical e)ciency of hydrogen production can be calculated theoretically, and is estimated to be 10% (on solar spectrum basis) for the photoheterotrophic process. With use of the theoretical photochemical e)ciency, and the climatic data on sunlight irradiance at a certain location at a certain moment of the year, the theoretical maximum hydrogen production can be estimated. Data on H2 yields and photochemical e)ciency from experiments reported in the literature are summarized. Photochemical e)ciencies, essentially based on artiÿcial light, can reach 10% or even more, but only at low light intensities, with associated low-H2 production rates. Some re<ections on possible photobioreactors lead to two types of (modiÿed) photobioreactors that might be successful for a large-scale biological hydrogen production. -

Biohydrogen Production by Photofermentation of Lactic Acid Using Thiocapsa Roseopersicina
U.P.B. Sci. Bull., Series B, Vol. 72, Iss. 2, 2010 ISSN 1454-2331 BIOHYDROGEN PRODUCTION BY PHOTOFERMENTATION OF LACTIC ACID USING THIOCAPSA ROSEOPERSICINA Éva HARAI (MOLNOS)1, Árpád KAPÁS2, Szabolcs LÁNYI3, Beáta ÁBRAHÁM4, Iosif NAGY5, Ovidiu MUNTEAN6 Hidrogenul obţinut prin procese biologice este denumit biohidrogen. Procesele biologice de producere a hidrogenului prezintă anumite avantaje, cum ar fi operare în condiţii blânde, selectivitate ridicată şi posibilitatea utilizării surselor regenerabile, dar au şi dezavantaje, precum eficienţa scăzută a conversiei şi costurile ridicate ale operării fotobioreactorului. Scopul nostru este studierea fenomenului de obţinere a biohidrogenului din acidul lactic, specific subproduselor industriei laptelui, prin fotofermentaţie cu bacterii fotosintetice. În această lucrare sunt prezentate caracteristicile definitorii ale bacteriilor Thiocapsa roseopersicina, precum şi acele puncte critice, pe care trebuie să se pună accentul în proiectarea şi construirea unui fotoreactor inovativ. The biologically produced hydrogen is defined as biohydrogen. Biological hydrogen production methods offer distinct advantages, such as operation under mild conditions, high selectivity and the possibility of using renewable sources, but have also some disadvantages, such as low conversion efficiency and high operating cost of photobioreactors. Our goal is to study photofermentative biohydrogen production with photosynthetic bacteria from lactic acid, also found in dairy wastewater. The focus of this paper is on the essential -

Producing Hydrogen in Sequential Dark and Photofermentation from Four Different Distillery Wastewaters
Pol. J. Environ. Stud. Vol. 29, No. 4 (2020), 2935-2944 DOI: 10.15244/pjoes/112062 ONLINE PUBLICATION DATE: 2020-03-02 Original Research Producing Hydrogen in Sequential Dark and Photofermentation from Four Different Distillery Wastewaters Roman Zagrodnik*, Krystyna Seifert, Mikołaj Stodolny, Anna Juszczak Faculty of Chemistry, Adam Mickiewicz University, Poznań, Poland Received: 1 August 2019 Accepted: 1 September 2019 Abstract Four different distillery wastewaters were applied as the substrates for dark and photofermentation – wheat stillage (WTL), wheat syrup (WSR), maize stillage (MTL) and maize syrups (MSR). WTL was found to be the most effective substrate in dark fermentation, with a hydrogen production of 0.88 L H2/Lmedium and a yield of 1.17 L H2/LWTL. In the photofermentation experiments, the highest hydrogen production (1.7 L H2/Lmedium) and the highest yield (8.6 L H2/LWSR) were obtained with WSR as the substrate. A sequential two-step treatment, comprising dark fermentation followed by photofermentation, was applied to the same four substrates. WTL and MTL were the substrates for dark fermentation and the effluent after 5-fold dilution was used as the substrate in photofermentation. The maximum hydrogen production in the two-step system was 4.47 L H2/LWTL. Simultaneously, the organic compounds from the waste and those formed in dark fermentation were completely utilized, except for ethanol and methanol, which were 19% and 10% utilized, respectively. Keywords: distillery wastewaters, dark fermentation, photofermentation, biohydrogen, two-step system Introduction was estimated that the world demand for ethyl alcohol would increase by 70% in the next 10 years because of Large-scale fermentative production of ethyl its application as a gasoline component [1]. -

Metabolic Flux Analysis for the Hydrogen Production Via Anaerobic Digestion and Photofermentation
METABOLIC FLUX ANALYSIS FOR THE HYDROGEN PRODUCTION VIA ANAEROBIC DIGESTION AND PHOTOFERMENTATION Victor Jurado, Axayacatl Gonzalez, Ines Garcia; Unidad Profesional Interdisciplinaria de Biotecnologia Del IPN, Bioprocesses Department, Mexico D.F. 07340; [email protected] Key words: MFA, Photo-fermentation, Dark-Fermentation Introduction. An alternative for the treatment constructed. Matrix dimensions were [23 x of organic wastes is the anaerobic digestion. 18], and it was defined as full-ranked. The This process leads to the production of system contains 4 degrees of freedom which hydrogen (H2) and methane, which have means that to evaluate the flux distribution 4 been identified as potential biofuels. Due to values should be known. To evaluate the its high energetic content, several attempts accuracy of the model, experimental data for increasing H2 production yields in dark obtained previously for the research group fermentation have been done. In recent years was employed. Since the number of the use of photoautotrophic bacteria that are experimentally measured rates was higher able to produce H2 has been explored since than the number of degrees of freedom these microorganisms can use organic acids required, different combinations were tested for growing [1]. for calculating the other production rates The mechanisms and regulation systems for (Table 1). Table 1. Gross Error between Experimental and H2 production are not completely understood due to the complexity of the metabolic Calculated Rates. DF indicates that the compound was used a degree of freedom. networks in dark and photo-fermentation Metabolite Measured Tests (Error, %) systems. To date, there are some metabolic Rates T1 T2 T3 T4 T5 (mol/h) models which attempt describing the Glucose -1.34x10-3 DF DF DF DF DF -4 metabolic changes during H2 production [2,3]. -

Enhancing Hydrogen Productivity of Photosynthetic Bacteria from the Formulated Carbon Components of Lignocellulose
Enhancing Hydrogen Productivity of Photosynthetic Bacteria from the Formulated Carbon Components of Lignocellulose Chuan Zhang ( [email protected] ) North China University of Water Resource and Electric Power Guihong Wang North China University of Water Resources and Electric Power Shuaishuai Ma North China University of Water Resources and Electric Power Hao Huang North China University of Water Resources and Electric Power Yixiao Ma North China University of Water Resources and Electric Power Zhaoran Li North China University of Water Resources and Electric Power Research Article Keywords: Photofermentative hydrogen production, lignocellulose, photosynthetic bacterial growth, nitrogenase activity, response surface methodology (RSM) Posted Date: July 16th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-708580/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Enhancing hydrogen productivity of photosynthetic bacteria from the formulated carbon components of lignocellulose Chuan Zhanga,b*, Guihong Wanga, Shuaishuai Maa, Hao Huanga, Yixiao Maa, Zhaoran Lia,b a School of Electric Power, North China University of Water Resource and Electric Power, No.36 Beihuan Road, Jinshui District, Zhengzhou, 450045, People’s Republic of China b Key Laboratory of Low-grad Energy Utilization Technologies and Systems (Ministry of Education), Chongqing Universtity, No. 174 Shazheng Street, Shapingba District, Chongqing, 400044, People’s Republic of China ABSTRACT To develop an efficient photofermentative -

Metabolic Engineering to Improve Biohydrogen Production by Rhodobacter Capsulatus JP91
Université de Montréal Metabolic Engineering to Improve Biohydrogen Production by Rhodobacter capsulatus JP91 Par Rajaa Sherteel Département de microbiologie, infectiologie et immunologie Faculté de Médecine Thèse présentée à la Faculté des Études Supérieures et Postdoctorales en vue de l’obtention du grade de MSc. en Microbiologie et Immunologie Avril, 2017 © Rajaa Sherteel, 2017 Université de Montréal Faculté des Études Supérieures Cette thèse intitulée: Metabolic Engineering to Improve Biohydrogen Production by Rhodobacter capsulatus JP91 Présentée par: Rajaa Sherteel A été évaluée par un jury composé des personnes suivantes: Dr. George Szatmari, Président-rapporteur Dr. Patrick C. Hallenbeck, Directeur de recherche Dr. Luke Masson, Membre du jury RÉSUMÉ La demande pour l'énergie augmente de jour en jour, ce qui se traduit par une attention mondiale à l'égard d'autres carburants respectueux de l'environnement parce que les combustibles fossiles nuisent à l'environnement. La production biologique d'hydrogène est une méthode alternative pour la production d'hydrogène, grâce à laquelle elle est produite dans des conditions douces, respectueux de l'environnement. La production photo-biologique d'hydrogène par les bactéries photosynthétiques pourpres non sulfureuses est un processus prometteur dans lequel les bactéries peuvent capturer de l'énergie lumineuse pour conduire la production d'H2 avec leur système de nitrogénase. Cependant, certaines voies métaboliques, tel que la fixation du CO2 et la biosynthèse du PHB, rivalisent avec la nitrogénase pour les électrons. Récemment, l'génie la métabolique a été appliqué pour améliorer le taux et le rendement de production d'H2. Le but de la présente étude était d'améliorer le rendement de la production d'H2 pendant la Photosynthèse par Rhodobacter capsulatus JP91 en utilisant des approches d'ingénierie métabolique. -

Improving Biohydrogen Productivity by Microbial Dark- and Photo- MARK Fermentations: Novel Data and Future Approaches ⁎ Karen Trchouniana,B, R
Renewable and Sustainable Energy Reviews 80 (2017) 1201–1216 Contents lists available at ScienceDirect Renewable and Sustainable Energy Reviews journal homepage: www.elsevier.com/locate/rser Improving biohydrogen productivity by microbial dark- and photo- MARK fermentations: Novel data and future approaches ⁎ Karen Trchouniana,b, R. Gary Sawersc, Armen Trchouniana,b, a Research Institute of Biology, Faculty of Biology, Yerevan State University, Armenia b Department of Biochemistry, Microbiology and Biotechnology, Faculty of Biology, Yerevan State University, 0025 Yerevan, Armenia c Institute of Biology/Microbiology, Martin-Luther University of Halle-Wittenberg, Halle (Saale), Germany ARTICLE INFO ABSTRACT Keywords: Hydrogen (H2)isaneffective, environmentally friendly and renewable source of fuel that can be produced Hydrogen production during dark- and photo-fermentation by different facultative and obligate anaerobic and purple bacteria and Dark- and photo-fermentation microalgae. This product is known as biohydrogen. It has the advantage of variable yield at low temperature (for Bacteria and microalgae mesophiles growing best at moderate temperature) and relatively low production cost, if compared with Hydrogenases and nitrogenases thermochemical methods. To develop fermentative H production biotechnology using cheap carbonaceous by- Glycerol 2 products and utilization of organic wastes, the selection or construction of effective bacterial strains and Carbon-containing wastes Renewable and sustainable energy optimization of technology process conditions are required. Here we review recent new data that have been obtained with Escherichia coli, Clostridium beijerinskii, Rhodobacter sphaeroides and other bacteria. Activities of [Ni-Fe]-hydrogenases of dark-fermentative bacteria and [Mo]-nitrogenase and [Ni-Fe]-hydrogenase of photo-fermentative species have been examined after growth with different carbon sources, using pure cultures, as well as co-culture and mixed-cultures technologies. -

Hybrid Strategies to Combine Dark Fermentation and Photo
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of Birmingham Research Archive, E-prints Repository Redwood et al. - Dual systems for Bio-H2 – Reviews in Environ. Sci. Bio/Technol. 8:149 1 http://dx.doi.org/10.1007/s11157-008-9144-9 Integrating dark and light bio-hydrogen production strategies: towards the hydrogen economy Mark D. Redwood, Marion Paterson-Beedle and Lynne E. Macaskie School of Biosciences, University of Birmingham Correspondence should be addressed to Dr Mark D. Redwood, School of Biosciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. Tel: +44 1214145889 Fax: +44 1214145925 e-mail: [email protected] Keywords: bio-hydrogen, bioenergy, renewable energy, hydrogen economy, dark fermentation, dual systems, photosynthesis. Abstract Biological methods of hydrogen production are preferable to chemical methods because of the possibility to use sunlight, CO2 and organic wastes as substrates for environmentally benign conversions, under moderate conditions. By combining different microorganisms with different capabilities, the individual strengths of each may be exploited and their weaknesses overcome. Mechanisms of bio-hydrogen production are described and strategies for their integration are discussed. Dual systems can be divided broadly into wholly light-driven systems (with microalgae/cyanobacteria as the 1st stage) and partially light-driven systems (with a dark, fermentative initial reaction). Review and evaluation of published data suggests that the latter type of system holds greater promise for industrial application. This is because the calculated land area required for a wholly light-driven dual system would be too large for either centralised (macro-) or decentralised (micro-)energy generation. -
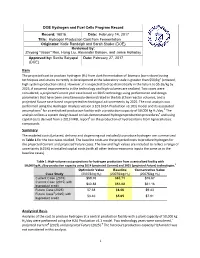
Hydrogen Production Cost from Fermentation Originator: Katie Randolph and Sarah Studer (DOE)
DOE Hydrogen and Fuel Cells Program Record Record: 16016 Date: February 14, 2017 Title: Hydrogen Production Cost f rom Fermentation Originator: Katie Randolph and Sarah Studer (DOE) Reviewed by: Zhiyong "Jason" Ren, Hong Liu, Alexander Beliaev, and Jamie Holladay Approved by: Sunita Satyapal Date: February 27, 2017 (DOE) Item The projected cost to produce hydrogen (H2) from dark fermentation of biomass (corn stover) using techniques and strains currently in development at the laboratory scale is greater than $50/kg1 (untaxed, high system production rates). However, it is expected to drop dramatically in the future to $5.65/kg by 2025, if assumed improvements in the technology and high volumes are realized. Two cases were considered, a projected Current year case based on 2015 technology using performance and design parameters that have been simultaneously demonstrated in the lab at low reactor volumes, and a projected Future case based on projected technological advancements by 2025. The cost analysis was performed using the Hydrogen Analysis version 3.101 (H2A Production v3.101) model and its associated 2 3 assumptions for a centralized production facility with a production capacity of 50,000 kg H2/day. The analysis utilizes a system design based on lab-demonstrated hydrogen production procedures4 and using capital costs derived from a 2013 NREL report5 on the production of hydrocarbons from lignocellulosic compounds. Summary The modeled costs (untaxed, delivery and dispensing not included) to produce hydrogen are summarized in Table 1 for the two cases studied. The baseline costs are the projected costs to produce hydrogen for the projected Current and projected Future cases.