Differential Freshwater Flagellate Community Response to Bacterial Prey with a Focus on Planktonic Betaproteobacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Report Is Also Available in Adobe
U.S. Department of the Interior U.S. Geological Survey Water Quality of Rob Roy Reservoir and Lake Owen, Albany County, and Granite Springs and Crystal Lake Reservoirs, Laramie County, Wyoming, 1997-98 By Kathy Muller Ogle1, David A. Peterson1, Bud Spillman2, and Rosie Padilla2 Water-Resources Investigations Report 99-4220 Prepared in cooperation with the Cheyenne Board of Public Utilities 1U.S. Geological Survey 2Cheyenne Board of Public Utilities Cheyenne, Wyoming 1999 U.S. Department of the Interior Bruce Babbitt, Secretary U.S. Geological Survey Charles G. Groat, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government For additional information write to: District Chief U.S. Geological Survey, WRD 2617 E. Lincolnway, Suite B Cheyenne, Wyoming 82001-5662 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286, Denver Federal Center Denver, Colorado 80225 Information regarding the programs of the Wyoming District is available on the Internet via the World Wide Web. You may connect to the Wyoming District Home Page using the Universal Resource Locator (URL): http://wy.water.usgs.gov CONTENTS Page Abstract ............................................................................................................................................................................... 1 Introduction........................................................................................................................................................................ -

Supporting Information
Supporting Information Parker et al. 10.1073/pnas.0806481105 Table S1. Species abbreviations used in Fig. 4A Major taxon Species Abbreviation Major taxon Species Abbreviation Dinobryon cylindraceum Dino cyli Diatoms Achnanthes flexella Achn flex Dinobryon sociale Dino soci Achnanthes lanceolata Dinobryon sp. (monad) Achnanthes minutissima Kephyrion boreale Keph bore Cocconeis placentula Kephyrion sp. Cyclotella atomus Cycl atom Mallomonas sp. Cyclotella bodanica Cycl boda Ochromonas minima Ochr mini Cyclotella comensis Cycl come Ochromonas sp. Cyclotella glomerata Pseudopedinella sp. Cyclotella ocellata Cycl ocel Salpingoeca sp. Cyclotella sp. Synura sp. Cymbella arctica Cymb arct Cymbella descripta Cymb desc Cryptophytes Cryptomonas erosa Cryp eros Cymbella minuta Cymb minu Cryptomonas marsonii Cryp mars Cymbella sp. Cryptomonas ovata Denticula subtilis Dent subt Cryptomonas platyrius Cryp plat Diatoma vulgare Diat vulg Cryptomonas reflexa Cryp refl Fragilaria capucina Frag capu Cryptomonas rostratiformis Cryp rost Fragilaria construens Cryptomonas sp. Fragilaria cyclopum Frag cycl Katablepharis ovalis Kata oval Fragilaria filiformis Rhodomonas lens Rhod lens Fragilaria tenera Rhodomonas minuta Rhod minu Fragilaria pinnata Frag pinn Gomphonema parvulum Gomp parv Dinoflagellates Amphidinium sp Gomphonema sp. Gymnodinium fungiforme Gymn fung Meridion circulare Meri circ Gymnodinium fuscum Navicula cryocephala Gymnodinium helvetica Gymn helv Navicula pupula Gymnodinium inversum Gymn inve Nitzschia perminuta Gymnodinium lacustre Gymn lacu Nitzschia -

An Integrative Approach Sheds New Light Onto the Systematics
www.nature.com/scientificreports OPEN An integrative approach sheds new light onto the systematics and ecology of the widespread ciliate genus Coleps (Ciliophora, Prostomatea) Thomas Pröschold1*, Daniel Rieser1, Tatyana Darienko2, Laura Nachbaur1, Barbara Kammerlander1, Kuimei Qian1,3, Gianna Pitsch4, Estelle Patricia Bruni4,5, Zhishuai Qu6, Dominik Forster6, Cecilia Rad‑Menendez7, Thomas Posch4, Thorsten Stoeck6 & Bettina Sonntag1 Species of the genus Coleps are one of the most common planktonic ciliates in lake ecosystems. The study aimed to identify the phenotypic plasticity and genetic variability of diferent Coleps isolates from various water bodies and from culture collections. We used an integrative approach to study the strains by (i) cultivation in a suitable culture medium, (ii) screening of the morphological variability including the presence/absence of algal endosymbionts of living cells by light microscopy, (iii) sequencing of the SSU and ITS rDNA including secondary structures, (iv) assessment of their seasonal and spatial occurrence in two lakes over a one‑year cycle both from morphospecies counts and high‑ throughput sequencing (HTS), and, (v) proof of the co‑occurrence of Coleps and their endosymbiotic algae from HTS‑based network analyses in the two lakes. The Coleps strains showed a high phenotypic plasticity and low genetic variability. The algal endosymbiont in all studied strains was Micractinium conductrix and the mutualistic relationship turned out as facultative. Coleps is common in both lakes over the whole year in diferent depths and HTS has revealed that only one genotype respectively one species, C. viridis, was present in both lakes despite the diferent lifestyles (mixotrophic with green algal endosymbionts or heterotrophic without algae). -

The Plankton Lifeform Extraction Tool: a Digital Tool to Increase The
Discussions https://doi.org/10.5194/essd-2021-171 Earth System Preprint. Discussion started: 21 July 2021 Science c Author(s) 2021. CC BY 4.0 License. Open Access Open Data The Plankton Lifeform Extraction Tool: A digital tool to increase the discoverability and usability of plankton time-series data Clare Ostle1*, Kevin Paxman1, Carolyn A. Graves2, Mathew Arnold1, Felipe Artigas3, Angus Atkinson4, Anaïs Aubert5, Malcolm Baptie6, Beth Bear7, Jacob Bedford8, Michael Best9, Eileen 5 Bresnan10, Rachel Brittain1, Derek Broughton1, Alexandre Budria5,11, Kathryn Cook12, Michelle Devlin7, George Graham1, Nick Halliday1, Pierre Hélaouët1, Marie Johansen13, David G. Johns1, Dan Lear1, Margarita Machairopoulou10, April McKinney14, Adam Mellor14, Alex Milligan7, Sophie Pitois7, Isabelle Rombouts5, Cordula Scherer15, Paul Tett16, Claire Widdicombe4, and Abigail McQuatters-Gollop8 1 10 The Marine Biological Association (MBA), The Laboratory, Citadel Hill, Plymouth, PL1 2PB, UK. 2 Centre for Environment Fisheries and Aquacu∑lture Science (Cefas), Weymouth, UK. 3 Université du Littoral Côte d’Opale, Université de Lille, CNRS UMR 8187 LOG, Laboratoire d’Océanologie et de Géosciences, Wimereux, France. 4 Plymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, UK. 5 15 Muséum National d’Histoire Naturelle (MNHN), CRESCO, 38 UMS Patrinat, Dinard, France. 6 Scottish Environment Protection Agency, Angus Smith Building, Maxim 6, Parklands Avenue, Eurocentral, Holytown, North Lanarkshire ML1 4WQ, UK. 7 Centre for Environment Fisheries and Aquaculture Science (Cefas), Lowestoft, UK. 8 Marine Conservation Research Group, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK. 9 20 The Environment Agency, Kingfisher House, Goldhay Way, Peterborough, PE4 6HL, UK. 10 Marine Scotland Science, Marine Laboratory, 375 Victoria Road, Aberdeen, AB11 9DB, UK. -

Biovolumes and Size-Classes of Phytoplankton in the Baltic Sea
Baltic Sea Environment Proceedings No.106 Biovolumes and Size-Classes of Phytoplankton in the Baltic Sea Helsinki Commission Baltic Marine Environment Protection Commission Baltic Sea Environment Proceedings No. 106 Biovolumes and size-classes of phytoplankton in the Baltic Sea Helsinki Commission Baltic Marine Environment Protection Commission Authors: Irina Olenina, Centre of Marine Research, Taikos str 26, LT-91149, Klaipeda, Lithuania Susanna Hajdu, Dept. of Systems Ecology, Stockholm University, SE-106 91 Stockholm, Sweden Lars Edler, SMHI, Ocean. Services, Nya Varvet 31, SE-426 71 V. Frölunda, Sweden Agneta Andersson, Dept of Ecology and Environmental Science, Umeå University, SE-901 87 Umeå, Sweden, Umeå Marine Sciences Centre, Umeå University, SE-910 20 Hörnefors, Sweden Norbert Wasmund, Baltic Sea Research Institute, Seestr. 15, D-18119 Warnemünde, Germany Susanne Busch, Baltic Sea Research Institute, Seestr. 15, D-18119 Warnemünde, Germany Jeanette Göbel, Environmental Protection Agency (LANU), Hamburger Chaussee 25, D-24220 Flintbek, Germany Slawomira Gromisz, Sea Fisheries Institute, Kollataja 1, 81-332, Gdynia, Poland Siv Huseby, Umeå Marine Sciences Centre, Umeå University, SE-910 20 Hörnefors, Sweden Maija Huttunen, Finnish Institute of Marine Research, Lyypekinkuja 3A, P.O. Box 33, FIN-00931 Helsinki, Finland Andres Jaanus, Estonian Marine Institute, Mäealuse 10 a, 12618 Tallinn, Estonia Pirkko Kokkonen, Finnish Environment Institute, P.O. Box 140, FIN-00251 Helsinki, Finland Iveta Ledaine, Inst. of Aquatic Ecology, Marine Monitoring Center, University of Latvia, Daugavgrivas str. 8, Latvia Elzbieta Niemkiewicz, Maritime Institute in Gdansk, Laboratory of Ecology, Dlugi Targ 41/42, 80-830, Gdansk, Poland All photographs by Finnish Institute of Marine Research (FIMR) Cover photo: Aphanizomenon flos-aquae For bibliographic purposes this document should be cited to as: Olenina, I., Hajdu, S., Edler, L., Andersson, A., Wasmund, N., Busch, S., Göbel, J., Gromisz, S., Huseby, S., Huttunen, M., Jaanus, A., Kokkonen, P., Ledaine, I. -
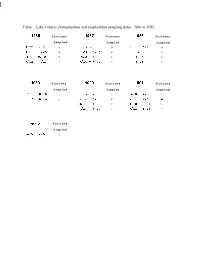
Table 1. Lake Ontario Phytoplankton and Zooplankton Sampling Dates, 1986 to 1992
Table 1. Lake Ontario phytoplankton and zooplankton sampling dates, 1986 to 1992. 1986 Stations 1987 Stations 1988 Stations Sampled Sampled Sampled 4/20 - 4/21 8 4/21 8 4/11 - 4/12 8 4/24 - 4/25 8 4/23 - 4/24 8 4/13 8 8/9 - 8/10 8 8/2 - 8/3 8 8/14 8 E/12 - E/13 8 8/14 - 8/15 8 8/16 8 1989 Stations 1990 Stations 1991 Stations Sampled Sampled Sampled 8/15 - 8/16 8 4/13 8 4/8 - 4/9 8 B/17 - 8/18 8 4/14 - 4/15 8 4/11 - 4/13 8 8/11 - 8/12 8 8/10 - B/11 8 8/13 - 8/14 8 E/12 - 8/13 8 1992 Stations Sampled 4/5 - 4/6 8 34 Table 2. Number of species observed in each algal division or grouping, Lake Ontario, 1986 to 1992. Spring and summer data only. BAC=Bacillariophyta, CHL=Chlorophyta, CHR=Chrysophyta, COL - colorless flagellates, CRY=Cryptophtya, CYA=Cyanophyta, EUG = Euglenophyta, PYR=Pyrrophyta, UN1 = unidentified flagellates. NUMBER OF SPECIES 1987 1988 1989 1990 1991 1992 1986-92 70 65 50 56 72 25 143 43 28 44 43 51 16 116 24 20 26 27 18 10 54 2 2 1 2 2 2 5 17 11 10 16 17 15 26 7 4 13 11 9 5 19 0 0 1 2 0 0 3 3 4 6 5 5 3 11 0 1 0 0 0 0 2 TOTAL 168 166 135 151 162 174 76 379 - -. -

Systema Naturae. the Classification of Living Organisms
Systema Naturae. The classification of living organisms. c Alexey B. Shipunov v. 5.601 (June 26, 2007) Preface Most of researches agree that kingdom-level classification of living things needs the special rules and principles. Two approaches are possible: (a) tree- based, Hennigian approach will look for main dichotomies inside so-called “Tree of Life”; and (b) space-based, Linnaean approach will look for the key differences inside “Natural System” multidimensional “cloud”. Despite of clear advantages of tree-like approach (easy to develop rules and algorithms; trees are self-explaining), in many cases the space-based approach is still prefer- able, because it let us to summarize any kinds of taxonomically related da- ta and to compare different classifications quite easily. This approach also lead us to four-kingdom classification, but with different groups: Monera, Protista, Vegetabilia and Animalia, which represent different steps of in- creased complexity of living things, from simple prokaryotic cell to compound Nature Precedings : doi:10.1038/npre.2007.241.2 Posted 16 Aug 2007 eukaryotic cell and further to tissue/organ cell systems. The classification Only recent taxa. Viruses are not included. Abbreviations: incertae sedis (i.s.); pro parte (p.p.); sensu lato (s.l.); sedis mutabilis (sed.m.); sedis possi- bilis (sed.poss.); sensu stricto (s.str.); status mutabilis (stat.m.); quotes for “environmental” groups; asterisk for paraphyletic* taxa. 1 Regnum Monera Superphylum Archebacteria Phylum 1. Archebacteria Classis 1(1). Euryarcheota 1 2(2). Nanoarchaeota 3(3). Crenarchaeota 2 Superphylum Bacteria 3 Phylum 2. Firmicutes 4 Classis 1(4). Thermotogae sed.m. 2(5). -

Protista (PDF)
1 = Astasiopsis distortum (Dujardin,1841) Bütschli,1885 South Scandinavian Marine Protoctista ? Dingensia Patterson & Zölffel,1992, in Patterson & Larsen (™ Heteromita angusta Dujardin,1841) Provisional Check-list compiled at the Tjärnö Marine Biological * Taxon incertae sedis. Very similar to Cryptaulax Skuja Laboratory by: Dinomonas Kent,1880 TJÄRNÖLAB. / Hans G. Hansson - 1991-07 - 1997-04-02 * Taxon incertae sedis. Species found in South Scandinavia, as well as from neighbouring areas, chiefly the British Isles, have been considered, as some of them may show to have a slightly more northern distribution, than what is known today. However, species with a typical Lusitanian distribution, with their northern Diphylleia Massart,1920 distribution limit around France or Southern British Isles, have as a rule been omitted here, albeit a few species with probable norhern limits around * Marine? Incertae sedis. the British Isles are listed here until distribution patterns are better known. The compiler would be very grateful for every correction of presumptive lapses and omittances an initiated reader could make. Diplocalium Grassé & Deflandre,1952 (™ Bicosoeca inopinatum ??,1???) * Marine? Incertae sedis. Denotations: (™) = Genotype @ = Associated to * = General note Diplomita Fromentel,1874 (™ Diplomita insignis Fromentel,1874) P.S. This list is a very unfinished manuscript. Chiefly flagellated organisms have yet been considered. This * Marine? Incertae sedis. provisional PDF-file is so far only published as an Intranet file within TMBL:s domain. Diplonema Griessmann,1913, non Berendt,1845 (Diptera), nec Greene,1857 (Coel.) = Isonema ??,1???, non Meek & Worthen,1865 (Mollusca), nec Maas,1909 (Coel.) PROTOCTISTA = Flagellamonas Skvortzow,19?? = Lackeymonas Skvortzow,19?? = Lowymonas Skvortzow,19?? = Milaneziamonas Skvortzow,19?? = Spira Skvortzow,19?? = Teixeiromonas Skvortzow,19?? = PROTISTA = Kolbeana Skvortzow,19?? * Genus incertae sedis. -

Marine Biological Laboratory) Data Are All from EST Analyses
TABLE S1. Data characterized for this study. rDNA 3 - - Culture 3 - etK sp70cyt rc5 f1a f2 ps22a ps23a Lineage Taxon accession # Lab sec61 SSU 14 40S Actin Atub Btub E E G H Hsp90 M R R T SUM Cercomonadida Heteromita globosa 50780 Katz 1 1 Cercomonadida Bodomorpha minima 50339 Katz 1 1 Euglyphida Capsellina sp. 50039 Katz 1 1 1 1 4 Gymnophrea Gymnophrys sp. 50923 Katz 1 1 2 Cercomonadida Massisteria marina 50266 Katz 1 1 1 1 4 Foraminifera Ammonia sp. T7 Katz 1 1 2 Foraminifera Ovammina opaca Katz 1 1 1 1 4 Gromia Gromia sp. Antarctica Katz 1 1 Proleptomonas Proleptomonas faecicola 50735 Katz 1 1 1 1 4 Theratromyxa Theratromyxa weberi 50200 Katz 1 1 Ministeria Ministeria vibrans 50519 Katz 1 1 Fornicata Trepomonas agilis 50286 Katz 1 1 Soginia “Soginia anisocystis” 50646 Katz 1 1 1 1 1 5 Stephanopogon Stephanopogon apogon 50096 Katz 1 1 Carolina Tubulinea Arcella hemisphaerica 13-1310 Katz 1 1 2 Cercomonadida Heteromita sp. PRA-74 MBL 1 1 1 1 1 1 1 7 Rhizaria Corallomyxa tenera 50975 MBL 1 1 1 3 Euglenozoa Diplonema papillatum 50162 MBL 1 1 1 1 1 1 1 1 8 Euglenozoa Bodo saltans CCAP1907 MBL 1 1 1 1 1 5 Alveolates Chilodonella uncinata 50194 MBL 1 1 1 1 4 Amoebozoa Arachnula sp. 50593 MBL 1 1 2 Katz lab work based on genomic PCRs and MBL (Marine Biological Laboratory) data are all from EST analyses. Culture accession number is ATTC unless noted. GenBank accession numbers for new sequences (including paralogs) are GQ377645-GQ377715 and HM244866-HM244878. -

Ecology and Genetic Analysis of Katablepharis CRE, A
Ecology and genetic analysis of Katablepharis CRE, a heterotrophic flagellate that ‘blooms’ in the Columbia River estuary during the spring Peter Kahn, Tawnya Peterson, Peter Zuber Center for Coastal Margin Observation and Prediction & Institute of Environmental Health, Oregon Health & Science University, Portland, OR [email protected] 1. Background 4. Spatial and temporal distribution of Katablepharis CRE in 2013 assessed through qPCR • Heterotrophic protists play important roles in the complex estuarine food web by shaping bacterial populations through predation, 1.2E+04 transferring prey carbon to higher trophic levels, and remineralization of nutrients such as nitrogen and phosphorus. • While difficulty in their identification using light microscopy has often led to heterotrophic protists being lumped into broad classifications, Washington 1.0E+04 there is a strong need to develop methods that increase the spatial and temporal resolution of observations of particular organisms in order to 8.0E+03 discover the drivers of population structure and ecological function. Estuary Mouth /s) • The Columbia River estuary contains large amounts of allochthonous detritus which is the primary source of organic matter driving ecosystem SATURN-03 North Channel 3 6.0E+03 Columbia River (m processes. This organic matter can fuel a high level of bacterial growth and productivity, which in turn can fuel propagation of grazing South Channel SATURN-04 4.0E+03 heterotrophic protists via the microbial loop. However, major heterotrophic protists (primary consumers and bacterivores) in the estuary are not well characterized. 2.0E+03 Oregon Dam Outflow Bonneville 2. Katablepharis sequences dominate mid-salinity SSU clone libraries during the spring Pacific Ocean 0.0E+00 2 miles 1-Jan 1-Mar 1-May 1-Jul 1-Sep 1-Nov Apicomplexa Cercozoa Chrysomonadida Ciliophora Dinophyceae (H) Dinophyceae (M) Katablepharidaceae Other A. -
Great Lakes Protozoan Community
Common Planktonic Protozoa in the Upper Great Lakes An Illustrated Guide Hunter J. Carrick Dept. of Biology and Great Lakes Center for Environmental Research and Education State University of New York College at Buffalo 1300 Elmwood Avenue Buffalo, New York 14222 Gary L. Fahnenstiel U.S. Dept. of Commerce Great Lakes Environmental Research Laboratory Lake Michigan Field Station 1431 Beach Street Muskegon, Michigan 49441 ( ACKNOWLEDGEMENTS------------~ We would like to thank the crew of the RIV Shenehon (B. Burns, J. Grimes, and D. Morse). J. Cavaletto, M. McCormick, and T. Patton assisted us in the field. Discussions with M.L. Pace, D. Scavia, E. Sherr, E.F. Stoermer, W.D. Taylor, and R.G. Wetzel were helpful. An earlier version of the manuscript was reviewed by P. Lavrentyev. This research was supported by an IAGLR scholarship award and a merit grant from the University of Michigan, School of Natural Resources. Support from The University of Florida Foundation, Inc. to C. L. Schelske sup ported HJC during part of the study. Technical editing provided by J. Hutchinson and C. Darnell. Design and electronic production by C. Darnell. This publication was funded in part by the Michigan Sea Grant College Program with funds from grant number NA89AA-D-SG083, from the National Sea Grant College Program, National Oceanic and Atmospheric Administration, U.S. Department of Com merce, and funds from the State of Michigan. The Michigan Sea Grant College Program is a cooperative program of the University of Michigan and Michigan State University. ( ii PLATES Plate I Figure 1. Desmarella sp. (epifluorescent, ca. -
Organisms of the Hubbard Brook Valley, New Hampshire
United States Department of Organisms of the Agriculture Forest Service Hubbard Brook Valley, Northeastern New Hampshire Research Station General Technical Report NE-257 Abstract Lists the organisms, both plant and animal, that have been identified by scientists engaged in multidisciplinary ecological research in the Hubbard Brook Valley, New Hampshire, during the past three decades. The Valley encompasses the Hubbard Brook Experimental Forest, Mirror Lake, and other areas within the White Mountain region of Grafton County. The species lists included in this report are relatively complete for vascular plants, amphibians, birds, fish, mammals, phytoplankton, and zooplankton, and partially complete for insects, molluscs, and nonvascular plants. The Compilers RICHARD T. HOLMES is professor of environmental biology and professor of biological sciences with the Department of Biological Sciences at Dartmouth College, Hanover, New Hampshire. GENE E. LIKENS is director and president of the Institute of Ecosystem Studies, Millbrook, New York. Manuscript received for publication 12 January 1998 Published by: For additional copies: USDA FOREST SERVICE USDA Forest Service 5 RADNOR CORP CTR SUITE 200 Publications Distribution RADNOR PA 19087-4585 359 Main Road Delaware, OH 43015 March 1999 Fax: (740)368-0152 Visit our homepage at: http://www.fs.fed.us/ne Contents Introduction .................................................................................................................................... 1 Hubbard Brook Ecosystem Study ..........................................................................................